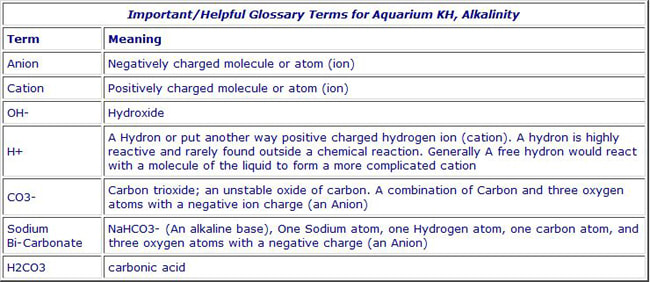
Aquarium Chemistry
Calcium | KH | GH | pH | Electrolytes (ions)
Calcium | KH | GH | pH | Electrolytes (ions)
Overview
Many aquarists overlook the need for electrolytes; positive mineral cations such as calcium & magnesium and the effect of KH (Carbonate hardness) in their freshwater aquarium (marine aquarium keepers tend to be more aware of these processes/parameters).
Minerals such calcium are essential for osmotic function in fish and many aquarists make the mistake of believing that some fish such as Discus or Bettas do not require Calcium or minerals when in reality (based on many studies in biochemistry and relating to Redox Balance) these mineral cations are essential and GH test kits to not give the full picture (Important, please see the GH section for more).
Unfortunately, based on many forum posts, client conversations, and emails; this aspect of aquarium keeping is one of the most misunderstood today as is often the case in the "The Aquarium Nitrogen Cycle" as well!
Reference:
• Aquarium Nitrogen Cycle
We as fish keepers should understand that fish will often adjust to poor electrolyte and calcium (& other necessary Redox reducing elements), however this does not prove this is best for the health of your fish any more than how fish will often adjust to aquariums that are crowded with infrequent water changes (which is also not good for long term fish health).
Aquariums that are overdue for a water change, with high bio load, lack mineral/electrolyte replenishment, or simply initially start out with incorrect chemistry often results in inadequate mineral and carbonate chemistry necessary for optimum bio function of the aquatic inhabitants (fish and invertebrates). Correction of this problem via water changes and/or addition of mineral or carbonate supplements may even show initial stress until the inhabitants adjust to the improved water quality.
Often aquarists in both fresh and sometimes even saltwater worry too much about pH while ignoring the importance of mineral cations/electrolytes (found in part via GH), and KH (more correctly identified as alkalinity, as it is in SW). As for pH, stability is more important than the actual pH number in freshwater.
In marine aquariums the actual pH number is of higher importance than in most FW aquariums (due to the fact here are much lower natural fluctuations in pH in natural bodies of saltwater), however even in saltwater a correct alkalinity (KH) is a must for proper buffering of marine pH and as well elements such as Calcium, Magnesium and Chloride are also very important to a healthy saltwater aquarium as you will read further in this article.
Popular but outdated aquarium traditions state that only certain fish need water high in electrolytes and calcium, this is only partly true. It is noteworthy that the Fish Body (as an organism) is alkaline by nature and is constantly attacked by free radicals which are usually acidic. While freshwater fish absorb needed H2O (saltwater drink constantly), the need for these electrolytes and calcium for some fish (such as Amazon River fish) is lower than some, however Proper Osmotic function still requires that calcium and other electrolytes be present in the aquatic environment as well as foods.
Reference:
• Fish Osmotic Function
This is why I have found that even fish such as Ram Cichlids thrive in a Balanced Reducing environment that has a Calcium level that often brings the GH over 150 ppm so as to provide positive mineral ions that are rapidly depleted in the closed system ANY aquarium is. This is also why the aquarium traditions of lower GH for certain fish are only half correct as they miss the aspect of balancing water with important mineral cations as well as with acid buffers. The result of incomplete mineral cations is often a fish that is more susceptible to disease (such as Columnaris) with shorter life spans.
Reference:
• A Healthy Aquarium; Disease Prevention
In particular this quote from the above article: "Bringing this back to an aquarium, if you immediately drive out all positive mineral ions in a mis-guided attempt to duplicate the Amazon environment, your fish will be constantly deprived of these essential mineral ions! This is analogous to a person avoiding all sun and then refusing to take any vitamin D supplements." The FACTS are just as all people need vitamin D, ALL FISH need positive mineral ions!!!
Often aquarium keepers utilize RO water (which is excellent when used correctly), but often fail to add anything more than immediate electrolytes. It is helpful to also add a more complete and often continuous supply of mineral cations to this RO water. More importantly, ALL RO water should have KH Buffers added or problems of water stability WILL be encountered. Worse, which should never be done, is the use of products that soften water by driving out mineral Cations or water from home water softeners which does the same. The result is water that CANNOT maintain essential mineral ions (even if added back) and a poor Redox balance!
This is a MUST Read article chapter on this subject of RO water use, including Buffers:
• Correct RO, DI Water Use in Aquariums
KH is basically the alkaline buffering capacity of your aquarium (there also is an opposite acid buffering process which when combined with KH is important for planted aquariums or low pH aquariums.
See pH/ Amazon River Section). A KH (Alkalinity) above 50 ppm helps prevent sudden drops in pH (although any KH will act to prevent sudden pH drops, but often lower levels do not handle large bio loads or large sudden changes).
KH (carbonate hardness) is an important source of energy for nitrifying bacteria that eliminate ammonia and nitrite. In addition, carbonates may be used by plants for photosynthesis when carbon dioxide (CO2) is absent.KH (Carbonate) buffering is especially important with Livebearers, Goldfish, East African Cichlids (Rift Lake), Brackish and many other freshwater fish (which should have an even higher KH over 100-150 or more).
In saltwater aquariums your KH (which is more correctly referred to as alkalinity) should be around 150-200 with as high as 240 ppm for high bio load closed system aquariums (which most aquariums area closed system).
Further Reference for marine/saltwater aquariums:
• General Marine Aquarium Water Parameters to maintain
The production of Nitrates (via processes that are similar to the production of nitric & carbonic acids) will slowly reduce your ph, but an adequate KH will keep a more stable ph. This is important to note, if your KH is low and your ph has been dropping, a large water change can cause stress on your fish, or even kill them (don't get me wrong, water changes are VERY important, just be careful with large water changes to correct very poor conditions).
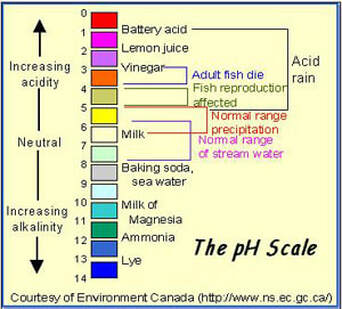
What is missed by many aquarists is that the pH scale is logarithmic, which means a 1 point drop/rise in pH is a tenfold change in acid or alkalinity (for more about this please read the pH section).
A proper electrolyte and calcium level, GH, & KH (by the term “proper” I mean what is the best level for the fish kept) can also have a positive effect on the aquarium Redox Balance, which recent studies have shown to be more important to fish and other animal health than pH. A positive mineral ion balance contributes to the Redox reduction potential of water, maintaining a more stable water environment, and the excess electrons attach themselves to free radicals, improving disease resistance.
For more information here, please reference this article:
• The Redox Potential/Balance in Aquariums and How it Relates to Aquatic Health
Minerals such calcium are essential for osmotic function in fish and many aquarists make the mistake of believing that some fish such as Discus or Bettas do not require Calcium or minerals when in reality (based on many studies in biochemistry and relating to Redox Balance) these mineral cations are essential and GH test kits to not give the full picture (Important, please see the GH section for more).
Unfortunately, based on many forum posts, client conversations, and emails; this aspect of aquarium keeping is one of the most misunderstood today as is often the case in the "The Aquarium Nitrogen Cycle" as well!
Reference:
• Aquarium Nitrogen Cycle
We as fish keepers should understand that fish will often adjust to poor electrolyte and calcium (& other necessary Redox reducing elements), however this does not prove this is best for the health of your fish any more than how fish will often adjust to aquariums that are crowded with infrequent water changes (which is also not good for long term fish health).
Aquariums that are overdue for a water change, with high bio load, lack mineral/electrolyte replenishment, or simply initially start out with incorrect chemistry often results in inadequate mineral and carbonate chemistry necessary for optimum bio function of the aquatic inhabitants (fish and invertebrates). Correction of this problem via water changes and/or addition of mineral or carbonate supplements may even show initial stress until the inhabitants adjust to the improved water quality.
Often aquarists in both fresh and sometimes even saltwater worry too much about pH while ignoring the importance of mineral cations/electrolytes (found in part via GH), and KH (more correctly identified as alkalinity, as it is in SW). As for pH, stability is more important than the actual pH number in freshwater.
In marine aquariums the actual pH number is of higher importance than in most FW aquariums (due to the fact here are much lower natural fluctuations in pH in natural bodies of saltwater), however even in saltwater a correct alkalinity (KH) is a must for proper buffering of marine pH and as well elements such as Calcium, Magnesium and Chloride are also very important to a healthy saltwater aquarium as you will read further in this article.
Popular but outdated aquarium traditions state that only certain fish need water high in electrolytes and calcium, this is only partly true. It is noteworthy that the Fish Body (as an organism) is alkaline by nature and is constantly attacked by free radicals which are usually acidic. While freshwater fish absorb needed H2O (saltwater drink constantly), the need for these electrolytes and calcium for some fish (such as Amazon River fish) is lower than some, however Proper Osmotic function still requires that calcium and other electrolytes be present in the aquatic environment as well as foods.
Reference:
• Fish Osmotic Function
This is why I have found that even fish such as Ram Cichlids thrive in a Balanced Reducing environment that has a Calcium level that often brings the GH over 150 ppm so as to provide positive mineral ions that are rapidly depleted in the closed system ANY aquarium is. This is also why the aquarium traditions of lower GH for certain fish are only half correct as they miss the aspect of balancing water with important mineral cations as well as with acid buffers. The result of incomplete mineral cations is often a fish that is more susceptible to disease (such as Columnaris) with shorter life spans.
Reference:
• A Healthy Aquarium; Disease Prevention
In particular this quote from the above article: "Bringing this back to an aquarium, if you immediately drive out all positive mineral ions in a mis-guided attempt to duplicate the Amazon environment, your fish will be constantly deprived of these essential mineral ions! This is analogous to a person avoiding all sun and then refusing to take any vitamin D supplements." The FACTS are just as all people need vitamin D, ALL FISH need positive mineral ions!!!
Often aquarium keepers utilize RO water (which is excellent when used correctly), but often fail to add anything more than immediate electrolytes. It is helpful to also add a more complete and often continuous supply of mineral cations to this RO water. More importantly, ALL RO water should have KH Buffers added or problems of water stability WILL be encountered. Worse, which should never be done, is the use of products that soften water by driving out mineral Cations or water from home water softeners which does the same. The result is water that CANNOT maintain essential mineral ions (even if added back) and a poor Redox balance!
This is a MUST Read article chapter on this subject of RO water use, including Buffers:
• Correct RO, DI Water Use in Aquariums
KH is basically the alkaline buffering capacity of your aquarium (there also is an opposite acid buffering process which when combined with KH is important for planted aquariums or low pH aquariums.
See pH/ Amazon River Section). A KH (Alkalinity) above 50 ppm helps prevent sudden drops in pH (although any KH will act to prevent sudden pH drops, but often lower levels do not handle large bio loads or large sudden changes).
KH (carbonate hardness) is an important source of energy for nitrifying bacteria that eliminate ammonia and nitrite. In addition, carbonates may be used by plants for photosynthesis when carbon dioxide (CO2) is absent.KH (Carbonate) buffering is especially important with Livebearers, Goldfish, East African Cichlids (Rift Lake), Brackish and many other freshwater fish (which should have an even higher KH over 100-150 or more).
In saltwater aquariums your KH (which is more correctly referred to as alkalinity) should be around 150-200 with as high as 240 ppm for high bio load closed system aquariums (which most aquariums area closed system).
Further Reference for marine/saltwater aquariums:
• General Marine Aquarium Water Parameters to maintain
The production of Nitrates (via processes that are similar to the production of nitric & carbonic acids) will slowly reduce your ph, but an adequate KH will keep a more stable ph. This is important to note, if your KH is low and your ph has been dropping, a large water change can cause stress on your fish, or even kill them (don't get me wrong, water changes are VERY important, just be careful with large water changes to correct very poor conditions).
What is missed by many aquarists is that the pH scale is logarithmic, which means a 1 point drop/rise in pH is a tenfold change in acid or alkalinity (for more about this please read the pH section).
A proper electrolyte and calcium level, GH, & KH (by the term “proper” I mean what is the best level for the fish kept) can also have a positive effect on the aquarium Redox Balance, which recent studies have shown to be more important to fish and other animal health than pH. A positive mineral ion balance contributes to the Redox reduction potential of water, maintaining a more stable water environment, and the excess electrons attach themselves to free radicals, improving disease resistance.
For more information here, please reference this article:
• The Redox Potential/Balance in Aquariums and How it Relates to Aquatic Health
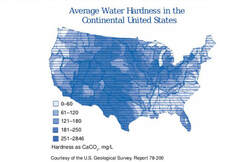
For those who use test kits that provide results in dKH or dGH, you can convert dH (German hardness) to ppm by multiplying your dH by 17.9.
To the left is a map of average water hardness (combined general and carbonate hardness) in the USA. Please click on the picture to enlarge).
PLEASE READ THE FULL ARTICLE below and referenced resources for a much more in-depth explanation of the OFTEN-MISUNDERSTOOD aspect of aquarium chemistry.
Please also note that product recommendations are based on controlled use, research, and recommendations by other trusted aquarium professionals; NOT because I work for SeaChem or any other company listed here. In other words, there MAY be other alternatives for the products listed here.
To the left is a map of average water hardness (combined general and carbonate hardness) in the USA. Please click on the picture to enlarge).
PLEASE READ THE FULL ARTICLE below and referenced resources for a much more in-depth explanation of the OFTEN-MISUNDERSTOOD aspect of aquarium chemistry.
Please also note that product recommendations are based on controlled use, research, and recommendations by other trusted aquarium professionals; NOT because I work for SeaChem or any other company listed here. In other words, there MAY be other alternatives for the products listed here.
KH Buffering
An important consideration of KH is that you can safely add the buffers (both freshwater and saltwater) that effect KH (Alkalinity) without sudden changes in chemistry, unless your freshwater KH is under 50 ppm (3 dKH) already. If your freshwater KH is under 50 ppm, pH bounces MAY be expected when first correcting a "too low" KH especially if using a carbonate such as "washing soda" or soda ash (a bicarbonate will not cause as much of a "bounce")
Once Stabilized, maintaining these KH (Alkaline) buffers keeps your tanks pH from drastic swings which can be deadly as once alkaline buffers are depleted, sudden and dangerous pH crashes are likely (see more about pH later in this article, including acid buffering).
Once Stabilized, maintaining these KH (Alkaline) buffers keeps your tanks pH from drastic swings which can be deadly as once alkaline buffers are depleted, sudden and dangerous pH crashes are likely (see more about pH later in this article, including acid buffering).
What is Buffering, both Alkaline (KH) and Acid? Alkalinity is the ability to resist change in pH on the measured addition of acid, meq/L (milli-equivalents per liter). An often-overlooked aspect of buffering is acidity, which is a measure of the water's ability to resist change in pH with the addition of base. Likewise, acidity should be expressed for what it is, the ability to resist change in pH with the measured addition of base, meq/L. Considered together, acidity and alkalinity constitute the buffer capacity of the water, the ability to resist change in pH from either direction.
The chemistry behind an alkaline buffer (KH) is quite complex, so I will not go into too much detail, however in the simplest terms I can think of; adding these carbonate (or bicarbonate) buffers will raise pH to a point of stability and the continued use of certain carbonate buffer “mixes” may raise pH even more. The rise in pH is related to the ratio of H+ to OH- ions.
The CO3- will react with the H+ and eliminate it. This reaction will cause more H20 to break up into H+ and OH- ions. Because some OH- ions were already present, this shifts the ratio thereby raising pH and making the water base alkaline. Putting it another way; KH (carbonate hardness or alkalinity) is caused by metals combined with a form of alkalinity: KH is the capacity of water to neutralize acids and KH is made up of compounds such as carbonate, bicarbonate, hydroxide, and sometimes borate & silicate. A higher KH can neutralize more acids produced from aquarium/pond biological processes than a lower KH.
More simply put: Maintaining a certain KH does not guarantee a certain pH due to many other chemistry aspects. However, maintaining a KH appropriate for the fish kept WILL prevent drastic pH swings!
In contrast, non-carbonate hardness (GH) forms when metals combine with anything other than alkalinity, which is why (despite many incorrect claims to the contrary) calcium does not raise pH directly. Carbonate hardness (KH) is sometimes called temporary hardness because it can be removed by boiling water. GH (non-carbonate hardness) by comparison cannot be broken down by boiling the water, so it is also known as permanent hardness.
Baking Soda (Sodium Bicarbonate NaHCO3-), is often used for KH, Sodium Bicarbonate will generally buffer at 8.0 to 8.2. Since pH = a measurement of H+ and the more H+ the lower the pH and less Alkalinity in short. Molar value wise, it takes twice as much as Bicarbonate as Carbonate to raise the Alkalinity up 1 Equilibrium unit. Volume wise it is 0.6 tsp of Bicarbonate vs. 0.4 tsp of Carbonate to raise the Alkalinity 1 milliequivalent (mEq) / or 2.8 dKH in 10 gals. Weight wise, is it is 3 grams vs. 2 grams. Due to that H, Bicarbonate has less impact on pH than Carbonate. Bicarbonate is mostly for raising the Alkalinity along with pH maintenance, while Carbonate is for raising the Alkalinity and pH.
Carbonate used only by itself should only be used if you have a low pH and Alkalinity. If it's to buffer up the Alkalinity, Bicarbonate is better. To stop the Sodium Carbonate ions from consuming too much H+ and to keep a pH of 7.0 we need to restrict the amount used, as it is always looking for H+ ions to consume. This is why I often prefer using products that not only contain sodium carbonates (or sodium bi carbonates), but the proper ratios of other minor elements such as Calcium and Magnesium.
The use of products such as Sea Chem Buffers; Marine, Gold, Malawi, Victoria for marine tanks and many freshwater tanks (such as livebearer, goldfish, rift lake cichlids) is an example of my preferred methods for KH/Alkalinity maintenance in these tanks. What is noteworthy is that all of the previously mentioned SeaChem buffers have basically the same formula, what differs is concentration and the amount you add to buffer to the desired alkalinity & pH. Aragonite and Seachem Cichlid Salts can also supply some carbonates as well.
Marine Aquarium alkalinity: Marine Buffer in particular will stabilize pH at 8.3 and no higher when used at full strength, assuming no problems with exceptional acid production such as decomposition in live rock. At lower doses it can be used in many freshwater applications. For marine reef aquariums, SeaChem Reef Builder is an excellent buffer that raises alkalinity without an immediate impact on pH. Over time this product will tend to stabilize pH at 8.3 Another advantage of Reef Builder is it is ionically balanced and WILL NOT deplete calcium, strontium, or magnesium which tend to precipitate out with increasing alkalinity, unlike what may happen with popular economy soda ash sodium carbonate products that have more immediate impact on pH (instead of bicarbonates).
Another popular method in Europe that unfortunately has not taken off in North America is the "Balling Method" which uses a 3-step program/method for complete reef aquarium chemical maintenance which includes alkalinity with step B. Step B uses sodium bicarbonate along with step A which uses calcium chloride dihydrate, along with step C with is complete sea salt minus the sodium chloride. The Balling method is scientifically proven and also maintains an ionic balance without any precipitation of key components of saltwater or rapid changes to pH. This method is also preferable for advanced reef keepers looking to move past bulk economy methods of marine reef maintenance.
Reference:
• Reef Aquarium Chemistry Maintenance Recommended read for further marine aquarium chemistry help.
Product Resources:
• SeaChem Reef Builder; Raises Carbonate Alkalinity
• TMC Bio-Calcium Original Balling Set; Parts A/B/C
Soft water or planted aquarium buffering: Soft water or planted aquariums (or lower pH Community aquariums) are best served by SeaChem Alkaline Buffer for still important KH/pH (about 50 ppm for softwater; 100 for low KH/pH community) stability that even Discus, Bettas, etc need, as pH fluctuations caused by lack of KH buffering can be harmful to these fish as well (since the pH scale is logarithmic, please see the pH section).
I recommend countering the KH Buffer with natural Acid Buffers such as Pillow Moss, Atison’s Spa, peat, or Driftwood etc. Or chemical Acid buffers such as SeaChem Acid Buffer, as a healthy lower pH has a “balanced equation” of both acid & alkaline buffers. Please note that the before mentioned "Natural" buffers often work very slowly as in many ways these counteract general hardness as much or more than carbonate hardness (KH). One or more of these buffers should also be employed for softwater aquariums for correct/balanced KH/pH chemistry (see the section later in this article dealing with Amazon River, SE Asia Water).
Product Resources:
• SeaChem Alkaline Buffer
• Pillow Moss; Natural Acid Buffer similar to Peat
• SeaChem Acid Buffer
SeaChem Alkaline Buffer is my preferred basic buffer (it is a non-phosphate buffer), especially in planted, softwater or community aquariums where baking soda can affect mineral equilibrium in a negative way and may not maintain a stable KH.
For freshwater aquariums (& basic marine aquariums), Wonder Shells are an excellent compliment for raising Calcium, magnesium, and electrolyte levels when used with Buffers, including Baking Soda.
However by themselves Wonder Shells (or similar mineral products such as Equilibrium) do not raise KH much and can actually cause KH stability issues if used incorrectly or a cure all for chemistry issues (as often incorrectly promoted by Weco, which is why sources selling this product based on Weco information should be avoided).
Product Resources:
• Wonder Shells; Mineral Blocks for Positive Mineral Ions
• Cichlid Salt; for Minerals, Sodium Chloride
Before I over promote AAP Wonder Shells in this article, while these can aid in proper water management, but they are not a magic bullet in any aquarium to make up for poor aquarium husbandry such as mulm build up under gravel or decorations. However, they can be one more piece of the water quality management puzzle and sometimes with fish such as Livebearers and Goldfish the results/benefits of use can be dramatic. As well, a Wonder Shell only aids in KH/pH stability, they cannot fix problems with these two parameters. In fact, incorrect use as often promoted by Weco can actually lower KH, which is why if you do use Wonder Shells, only purchase the FRESH AAP Wonder Shells, not the clearance stock with incorrect directions sold elsewhere!
I should also note that I do NOT find the use of Neutral pH regulators helpful for community aquariums (ditto for Discus Buffer). A more natural balance of carbonate buffers and acid buffers (or a mix both natural or supplemented buffers) is much better for long-term keeping of a healthy aquarium chemistry equilibrium. These products often use phosphates which are not a healthy way to neutralize pH/KH, and in fact these products drive out ESSENTIAL calcium and magnesium ions!!! As SeaChem states on their website: "Softens water by precipitating calcium and magnesium".
Dangers of long-term use includes negative affect on Redox Balance. As well, and this is backed up by the basic science of how these Neutral Regulators work along with the importance of calcium & magnesium for ALL fish, is that fish health IS AFFECTED long term.
If an aquarium keeper finds a neutral regulator the only way to stabilize an aquarium pH/KH, this indicates that there are likely too many acid producing organics such as mulm in canister filters (especially in ceramic media) or under gravel, decor, etc. in the aquarium. Another proof of this unnatural stability is if a Wonder Shell is used, it will produce a "dust" on the bottom that is easily stirred into a cloud in the water (due to phosphates in these products).
The bottom line is the use of Neutral Regulators or Discus Buffer such as by SeaChem or API Proper pH 7 is not a healthy nor natural way to maintain good aquarium chemistry and my years of maintaining many 100's of aquariums has born this out (This is not a knock on either company, in particular SeaChem, as most of their products I would highly recommend)!
The chemistry behind an alkaline buffer (KH) is quite complex, so I will not go into too much detail, however in the simplest terms I can think of; adding these carbonate (or bicarbonate) buffers will raise pH to a point of stability and the continued use of certain carbonate buffer “mixes” may raise pH even more. The rise in pH is related to the ratio of H+ to OH- ions.
The CO3- will react with the H+ and eliminate it. This reaction will cause more H20 to break up into H+ and OH- ions. Because some OH- ions were already present, this shifts the ratio thereby raising pH and making the water base alkaline. Putting it another way; KH (carbonate hardness or alkalinity) is caused by metals combined with a form of alkalinity: KH is the capacity of water to neutralize acids and KH is made up of compounds such as carbonate, bicarbonate, hydroxide, and sometimes borate & silicate. A higher KH can neutralize more acids produced from aquarium/pond biological processes than a lower KH.
More simply put: Maintaining a certain KH does not guarantee a certain pH due to many other chemistry aspects. However, maintaining a KH appropriate for the fish kept WILL prevent drastic pH swings!
In contrast, non-carbonate hardness (GH) forms when metals combine with anything other than alkalinity, which is why (despite many incorrect claims to the contrary) calcium does not raise pH directly. Carbonate hardness (KH) is sometimes called temporary hardness because it can be removed by boiling water. GH (non-carbonate hardness) by comparison cannot be broken down by boiling the water, so it is also known as permanent hardness.
Baking Soda (Sodium Bicarbonate NaHCO3-), is often used for KH, Sodium Bicarbonate will generally buffer at 8.0 to 8.2. Since pH = a measurement of H+ and the more H+ the lower the pH and less Alkalinity in short. Molar value wise, it takes twice as much as Bicarbonate as Carbonate to raise the Alkalinity up 1 Equilibrium unit. Volume wise it is 0.6 tsp of Bicarbonate vs. 0.4 tsp of Carbonate to raise the Alkalinity 1 milliequivalent (mEq) / or 2.8 dKH in 10 gals. Weight wise, is it is 3 grams vs. 2 grams. Due to that H, Bicarbonate has less impact on pH than Carbonate. Bicarbonate is mostly for raising the Alkalinity along with pH maintenance, while Carbonate is for raising the Alkalinity and pH.
Carbonate used only by itself should only be used if you have a low pH and Alkalinity. If it's to buffer up the Alkalinity, Bicarbonate is better. To stop the Sodium Carbonate ions from consuming too much H+ and to keep a pH of 7.0 we need to restrict the amount used, as it is always looking for H+ ions to consume. This is why I often prefer using products that not only contain sodium carbonates (or sodium bi carbonates), but the proper ratios of other minor elements such as Calcium and Magnesium.
The use of products such as Sea Chem Buffers; Marine, Gold, Malawi, Victoria for marine tanks and many freshwater tanks (such as livebearer, goldfish, rift lake cichlids) is an example of my preferred methods for KH/Alkalinity maintenance in these tanks. What is noteworthy is that all of the previously mentioned SeaChem buffers have basically the same formula, what differs is concentration and the amount you add to buffer to the desired alkalinity & pH. Aragonite and Seachem Cichlid Salts can also supply some carbonates as well.
Marine Aquarium alkalinity: Marine Buffer in particular will stabilize pH at 8.3 and no higher when used at full strength, assuming no problems with exceptional acid production such as decomposition in live rock. At lower doses it can be used in many freshwater applications. For marine reef aquariums, SeaChem Reef Builder is an excellent buffer that raises alkalinity without an immediate impact on pH. Over time this product will tend to stabilize pH at 8.3 Another advantage of Reef Builder is it is ionically balanced and WILL NOT deplete calcium, strontium, or magnesium which tend to precipitate out with increasing alkalinity, unlike what may happen with popular economy soda ash sodium carbonate products that have more immediate impact on pH (instead of bicarbonates).
Another popular method in Europe that unfortunately has not taken off in North America is the "Balling Method" which uses a 3-step program/method for complete reef aquarium chemical maintenance which includes alkalinity with step B. Step B uses sodium bicarbonate along with step A which uses calcium chloride dihydrate, along with step C with is complete sea salt minus the sodium chloride. The Balling method is scientifically proven and also maintains an ionic balance without any precipitation of key components of saltwater or rapid changes to pH. This method is also preferable for advanced reef keepers looking to move past bulk economy methods of marine reef maintenance.
Reference:
• Reef Aquarium Chemistry Maintenance Recommended read for further marine aquarium chemistry help.
Product Resources:
• SeaChem Reef Builder; Raises Carbonate Alkalinity
• TMC Bio-Calcium Original Balling Set; Parts A/B/C
Soft water or planted aquarium buffering: Soft water or planted aquariums (or lower pH Community aquariums) are best served by SeaChem Alkaline Buffer for still important KH/pH (about 50 ppm for softwater; 100 for low KH/pH community) stability that even Discus, Bettas, etc need, as pH fluctuations caused by lack of KH buffering can be harmful to these fish as well (since the pH scale is logarithmic, please see the pH section).
I recommend countering the KH Buffer with natural Acid Buffers such as Pillow Moss, Atison’s Spa, peat, or Driftwood etc. Or chemical Acid buffers such as SeaChem Acid Buffer, as a healthy lower pH has a “balanced equation” of both acid & alkaline buffers. Please note that the before mentioned "Natural" buffers often work very slowly as in many ways these counteract general hardness as much or more than carbonate hardness (KH). One or more of these buffers should also be employed for softwater aquariums for correct/balanced KH/pH chemistry (see the section later in this article dealing with Amazon River, SE Asia Water).
Product Resources:
• SeaChem Alkaline Buffer
• Pillow Moss; Natural Acid Buffer similar to Peat
• SeaChem Acid Buffer
SeaChem Alkaline Buffer is my preferred basic buffer (it is a non-phosphate buffer), especially in planted, softwater or community aquariums where baking soda can affect mineral equilibrium in a negative way and may not maintain a stable KH.
For freshwater aquariums (& basic marine aquariums), Wonder Shells are an excellent compliment for raising Calcium, magnesium, and electrolyte levels when used with Buffers, including Baking Soda.
However by themselves Wonder Shells (or similar mineral products such as Equilibrium) do not raise KH much and can actually cause KH stability issues if used incorrectly or a cure all for chemistry issues (as often incorrectly promoted by Weco, which is why sources selling this product based on Weco information should be avoided).
Product Resources:
• Wonder Shells; Mineral Blocks for Positive Mineral Ions
• Cichlid Salt; for Minerals, Sodium Chloride
Before I over promote AAP Wonder Shells in this article, while these can aid in proper water management, but they are not a magic bullet in any aquarium to make up for poor aquarium husbandry such as mulm build up under gravel or decorations. However, they can be one more piece of the water quality management puzzle and sometimes with fish such as Livebearers and Goldfish the results/benefits of use can be dramatic. As well, a Wonder Shell only aids in KH/pH stability, they cannot fix problems with these two parameters. In fact, incorrect use as often promoted by Weco can actually lower KH, which is why if you do use Wonder Shells, only purchase the FRESH AAP Wonder Shells, not the clearance stock with incorrect directions sold elsewhere!
I should also note that I do NOT find the use of Neutral pH regulators helpful for community aquariums (ditto for Discus Buffer). A more natural balance of carbonate buffers and acid buffers (or a mix both natural or supplemented buffers) is much better for long-term keeping of a healthy aquarium chemistry equilibrium. These products often use phosphates which are not a healthy way to neutralize pH/KH, and in fact these products drive out ESSENTIAL calcium and magnesium ions!!! As SeaChem states on their website: "Softens water by precipitating calcium and magnesium".
Dangers of long-term use includes negative affect on Redox Balance. As well, and this is backed up by the basic science of how these Neutral Regulators work along with the importance of calcium & magnesium for ALL fish, is that fish health IS AFFECTED long term.
If an aquarium keeper finds a neutral regulator the only way to stabilize an aquarium pH/KH, this indicates that there are likely too many acid producing organics such as mulm in canister filters (especially in ceramic media) or under gravel, decor, etc. in the aquarium. Another proof of this unnatural stability is if a Wonder Shell is used, it will produce a "dust" on the bottom that is easily stirred into a cloud in the water (due to phosphates in these products).
The bottom line is the use of Neutral Regulators or Discus Buffer such as by SeaChem or API Proper pH 7 is not a healthy nor natural way to maintain good aquarium chemistry and my years of maintaining many 100's of aquariums has born this out (This is not a knock on either company, in particular SeaChem, as most of their products I would highly recommend)!
Alkaline KH Buffers
Explanation of common Buffers used to raise Alkalinity (KH & eventually pH) Baking Soda (NaHCO3): Is essentially just Sodium bicarbonate and will raise KH, but it can easily be overdosed and does not always maintain as stable a KH or pH.
Sea Chem Marine Buffer: This is multi-ingredient product that not only raises KH and pH, but also GH as it is very balanced in its mineral balance. Due to the ingredients contained there in it will NOT raise pH past 8.3- 8.4 even when over dosed. When use in FW, small amounts should be used so as to slowly raised pH and KH as if overdosed you can raise pH to 8.3.
Product Resource:
• SeaChem Marine Buffer
SeaChem Malawi/Victoria Buffer: Is a blend of carbonates designed to increase carbonate hardness, buffer capacity and pH. It will also not raise pH past 8.4, even when overdosed (as with Marine Buffer, I have used this product for many freshwater applications such as Livebearers & Goldfish).
Product Resource:
SeaChem Malawi/Victoria Buffer; 7.7 to 8.4 Alkaline Buffer
SeaChem Tanganyika Buffer: Again, a similar product to MMalawi Buffer with additional necessary minerals & buffer capacity, however it will raise pH to 9.0 when used full strength.
SeaChem Gold Buffer: This once again is basically the same formula as the previously noted buffers, just the concentrations differ slightly as does the amount you would use. One can easily substitute Malawi Buffer and simply use less for goldfish or livebearers. What is also noteworthy is many based on poor Amazon Review Information, believe this is a product you simply add and it magically buffers to 7.8 PH, when in fact, as with all buffers you have to add a specific amount to match your unique aquarium environment!
SeaChem Alkaline Buffer: This is more straight forward KH buffer that has less added minerals when these are not desired (often by planted or soft water aquarium keepers). Alkaline Buffer will continue to increase pH to 7.8 (or as high as 8.5 with correct usage), however it still is more stable and moves pH much less dramatically than baking soda making it still a much better choice in FW. Alkaline Buffer is the preferred method (combined with Acid Buffer) for use with stabilizing Reverse Osmosis Water in lower pH/soft water aquariums. Please see the Acid/Alkaline Buffer Chart in the Low PH Buffers Section (next section) below for correct ratios.
Product Resources:
• SeaChem Alkaline Buffer
• TMC RO Water Filters for Advanced Aquarium Keepers
Sea Chem Marine Buffer: This is multi-ingredient product that not only raises KH and pH, but also GH as it is very balanced in its mineral balance. Due to the ingredients contained there in it will NOT raise pH past 8.3- 8.4 even when over dosed. When use in FW, small amounts should be used so as to slowly raised pH and KH as if overdosed you can raise pH to 8.3.
Product Resource:
• SeaChem Marine Buffer
SeaChem Malawi/Victoria Buffer: Is a blend of carbonates designed to increase carbonate hardness, buffer capacity and pH. It will also not raise pH past 8.4, even when overdosed (as with Marine Buffer, I have used this product for many freshwater applications such as Livebearers & Goldfish).
Product Resource:
SeaChem Malawi/Victoria Buffer; 7.7 to 8.4 Alkaline Buffer
SeaChem Tanganyika Buffer: Again, a similar product to MMalawi Buffer with additional necessary minerals & buffer capacity, however it will raise pH to 9.0 when used full strength.
SeaChem Gold Buffer: This once again is basically the same formula as the previously noted buffers, just the concentrations differ slightly as does the amount you would use. One can easily substitute Malawi Buffer and simply use less for goldfish or livebearers. What is also noteworthy is many based on poor Amazon Review Information, believe this is a product you simply add and it magically buffers to 7.8 PH, when in fact, as with all buffers you have to add a specific amount to match your unique aquarium environment!
SeaChem Alkaline Buffer: This is more straight forward KH buffer that has less added minerals when these are not desired (often by planted or soft water aquarium keepers). Alkaline Buffer will continue to increase pH to 7.8 (or as high as 8.5 with correct usage), however it still is more stable and moves pH much less dramatically than baking soda making it still a much better choice in FW. Alkaline Buffer is the preferred method (combined with Acid Buffer) for use with stabilizing Reverse Osmosis Water in lower pH/soft water aquariums. Please see the Acid/Alkaline Buffer Chart in the Low PH Buffers Section (next section) below for correct ratios.
Product Resources:
• SeaChem Alkaline Buffer
• TMC RO Water Filters for Advanced Aquarium Keepers
Acid Buffers
Explanation of common Acid Buffers used to counter a Base (Alkalinity): Bisulfate salts are the preferred acid buffer for planted aquariums or for very hard water where phosphate buffers may pose an algae or cloudiness problem.
Product Resource:
• SeaChem Acid Buffer; Bisulfate salts
Phosphoric Acid: Natural Acid Buffers which often contain Tannic Acid such as: Peat, Pillow Moss (Frog Moss), most Driftwood, Indian Almond Leaves.
Product Resources:
• Pillow/Frog Moss; Natural Aquarium Low pH Buffer
• Aquarium Driftwood
Tips for use of Buffers (Acid or Alkaline): I strongly suggest dissolving powdered buffers in warm to mildly hot water prior to addition to the aquarium. DO NOT MIX acid and alkaline buffers in the same container prior to introducing to the aquarium unless in large container such as a 5-gallon bucket meant as replacement water for a water change. Then add this solution slowly into the tank, sometimes in cases where KH is considerably depleted; this solution should be added in increments over a couple of hours if large adjustments need to be made (such a 4dKH ppm to 8dKH).
Always know your water source Buffering Capacity; Alkalinity (KH) prior to addition to your aquarium.
For instance, if your tap or well water has a KH of 200 and your desire is to maintain a KH of 200; NO ADDITIONAL KH buffer is needed at the time of water addition/change. However, with this same aquarium situation, if the KH drops between water changes, maintenance amounts of Buffers will need to be added (the amount is something you will need to determine via "trial & error" based on aquarium size and depletion rate).
Know your aquarium: As an example, if your KH is low (under 50 ppm) in say a Discus aquarium yet with little acid buffering; when you seek to bring up your aquarium KH for more stability, you can immediately "bounce" your pH. Acid buffering can be from natural sources, but whether natural or added chemically, if low when you make even small changes to your KH it can move a pH. So do NOT chase your pH! Find a pH that you can keep stable with the minimum KH and stick with it, whether it be 6.8 or 7.3 (as examples).
For more tips see this section near the end of this article:
• Quick Tips for Adding Buffers, etc.
Crushed Coral/Aragonite: Aragonite, crushed coral, or oyster shell are sometimes employed for KH and GH stabilization, however aragonite and more so crushed coral & oyster shell (as with Wonder Shells) only aid to stabilize KH (they are poor buffers, especially crushed coral) and should not be used in place of a true KH buffer such as Sea Chem Alkaline Buffer when true buffering is necessary due to fluctuating KH or pH whatever the cause may be.
In fact way back in the early 1980s, there was a fad to use Oyster shells as a substrate in marine aquariums, not only did I find this use not only perform a poor job at maintaining alkalinity, mineral content was also not aided even as well as crushed coral (further investigation and experiments showed part of the problem was that oyster shell tended to pack down much more than crushed coral and allowed for organics to be trapped and decompose increasing acids far faster than the oyster shell could buffer).
Crushed Coral (as with seashells and coral) is primarily made up of Calcium Carbonates (CaCO3) and has VERY LITTLE bicarbonates while Aragonite is of similar make up but has a much better surface area for dissolving of minerals making it a better choice of the two (especially when used in a reactor). With Seashells/coral, the surface area is lower yet, even if in high flow areas of an aquarium and thus provide poor buffering if any (based on many studies in marine aquariums I have performed over the years)
Some Aragonites (that have high carbonate content) are useful at stabilizing a higher kH of around 240 ppm, which is the maximum KH (alkalinity) needed for Marine Aquariums but does not respond to changes rapidly enough when carbonic acids are produced at a rapid rate in an aquarium (usually a high bio load or large amounts of organic mulm will cause this).
Even in marine aquariums with aragonite, this may not always be enough to maintain a proper alkalinity (KH) level, especially in tanks with high bio loads and without adequate water changes (even skimming can remove some elements). Aragonite generally has a few more essential minerals in higher concentrations such as the important mineral (for corals), Strontium.
Further Reference:
• Aquarium Bio Load
It often takes copious amounts of acids to free these minerals and what little bicarbonates/carbonates that are available (which is where a Calcium Reactor MAY be helpful in marine aquariums). For this reason, the use of crushed coral is more effective in a “Filter Bag” to release these minerals when used in Freshwater, especially African Cichlid aquaria. The use of a filter bag in a high flow area will improve the dissolution rate releasing more minerals and allowing for some buffering (as well as slightly improved adding mineral cations), although again I will point out not much carbonate (KH) buffering due to the mineral make up of crushed coral (Aragonite will do a slightly better buffering job when employed in a filter bag).
This said, despite the popular use of crushed coral/Aragonite for pH/KH control in African Cichlids, it is a poor choice for this, especially in high bio load aquariums due to the FACT of its mineral make up.
Simply put, you CANNOT make a mineral appear out of nowhere that does not exist and that seems to be what many mistakenly believe when using crushed coral to increase KH/pH. This is an unfortunate “aquatic myth” that many forums still perpetuate when a quick search of the chemical makeup of crushed coral would expose this folly.
And for those who use this equation to state otherwise: H2O + CO2 + CaCO3 <> Ca + 2HCO3, let me point out that this is great when used in a reaction chamber, but in the real world of an aquarium, I've yet to find that provides much buffering, including in marine aquariums with copious amounts of crushed coral. I still had to add additional buffers! Sorry actual experience with 1000s of aquariums trumps here!
The bottom line is that Crushed Coral MAY help maintain KH/pH in a low bio load aquarium, they are best used for minerals (GH). Even then, Wonder Shells (or similar mineral ion supplements such as AAP/SeaChem Replenish or Fresh Trace used regularly or in drip) are far superior to Crushed Coral due to the fact a Wonder Shell dissolves at a much faster rate and reacts much quicker to chemistry changes in the water than does crushed coral. As a well the use of a slow drip liquid mineral replenisher would also be superior to the use of Crushed Coral for mineral depletion. Besides the simple mineral makeup of crushed coral, my own extensive tests show that its use to increase KH as well as GH (Calcium and other minerals) simply is poor.
Product Resource:
• AAP Wonder Shells
• SeaChem Replenish; Can be added directly or by drip
Reference:
• The Mineral Argonite
In lower pH community tanks (6.8 to 7.4) KH (Alkaline) buffers such as Sea Chem Alkaline Buffer are still important, however, as noted earlier, I like to counter these with natural lower (acid) pH “buffers” such as Indian Almond Leaves, Peat, Pillow/Frog Moss, and/or Mango/Driftwood.
Buffering your freshwater aquarium is especially important if you have plants fed by CO2 which will raise pH during peak growth times, and there is scientific evidence that GH plays a role here as well.
Please see this article for more about this subject:
• AQUARIUM PLANTS; PROPER NUTRIENTS
For low pH/Soft Water Aquariums (such as Discus, Ram Cichlid, Betta), besides the before mentioned “slow” acid buffers (peat, Frog Moss, etc.), you can use immediate acid buffers with KH (carbonate) buffers for quicker results, such as SeaChem’s Acid Buffer.
Product Resource:
• SeaChem Acid Buffer; Bisulfate salts
Phosphoric Acid: Natural Acid Buffers which often contain Tannic Acid such as: Peat, Pillow Moss (Frog Moss), most Driftwood, Indian Almond Leaves.
Product Resources:
• Pillow/Frog Moss; Natural Aquarium Low pH Buffer
• Aquarium Driftwood
Tips for use of Buffers (Acid or Alkaline): I strongly suggest dissolving powdered buffers in warm to mildly hot water prior to addition to the aquarium. DO NOT MIX acid and alkaline buffers in the same container prior to introducing to the aquarium unless in large container such as a 5-gallon bucket meant as replacement water for a water change. Then add this solution slowly into the tank, sometimes in cases where KH is considerably depleted; this solution should be added in increments over a couple of hours if large adjustments need to be made (such a 4dKH ppm to 8dKH).
Always know your water source Buffering Capacity; Alkalinity (KH) prior to addition to your aquarium.
For instance, if your tap or well water has a KH of 200 and your desire is to maintain a KH of 200; NO ADDITIONAL KH buffer is needed at the time of water addition/change. However, with this same aquarium situation, if the KH drops between water changes, maintenance amounts of Buffers will need to be added (the amount is something you will need to determine via "trial & error" based on aquarium size and depletion rate).
Know your aquarium: As an example, if your KH is low (under 50 ppm) in say a Discus aquarium yet with little acid buffering; when you seek to bring up your aquarium KH for more stability, you can immediately "bounce" your pH. Acid buffering can be from natural sources, but whether natural or added chemically, if low when you make even small changes to your KH it can move a pH. So do NOT chase your pH! Find a pH that you can keep stable with the minimum KH and stick with it, whether it be 6.8 or 7.3 (as examples).
For more tips see this section near the end of this article:
• Quick Tips for Adding Buffers, etc.
Crushed Coral/Aragonite: Aragonite, crushed coral, or oyster shell are sometimes employed for KH and GH stabilization, however aragonite and more so crushed coral & oyster shell (as with Wonder Shells) only aid to stabilize KH (they are poor buffers, especially crushed coral) and should not be used in place of a true KH buffer such as Sea Chem Alkaline Buffer when true buffering is necessary due to fluctuating KH or pH whatever the cause may be.
In fact way back in the early 1980s, there was a fad to use Oyster shells as a substrate in marine aquariums, not only did I find this use not only perform a poor job at maintaining alkalinity, mineral content was also not aided even as well as crushed coral (further investigation and experiments showed part of the problem was that oyster shell tended to pack down much more than crushed coral and allowed for organics to be trapped and decompose increasing acids far faster than the oyster shell could buffer).
Crushed Coral (as with seashells and coral) is primarily made up of Calcium Carbonates (CaCO3) and has VERY LITTLE bicarbonates while Aragonite is of similar make up but has a much better surface area for dissolving of minerals making it a better choice of the two (especially when used in a reactor). With Seashells/coral, the surface area is lower yet, even if in high flow areas of an aquarium and thus provide poor buffering if any (based on many studies in marine aquariums I have performed over the years)
Some Aragonites (that have high carbonate content) are useful at stabilizing a higher kH of around 240 ppm, which is the maximum KH (alkalinity) needed for Marine Aquariums but does not respond to changes rapidly enough when carbonic acids are produced at a rapid rate in an aquarium (usually a high bio load or large amounts of organic mulm will cause this).
Even in marine aquariums with aragonite, this may not always be enough to maintain a proper alkalinity (KH) level, especially in tanks with high bio loads and without adequate water changes (even skimming can remove some elements). Aragonite generally has a few more essential minerals in higher concentrations such as the important mineral (for corals), Strontium.
Further Reference:
• Aquarium Bio Load
It often takes copious amounts of acids to free these minerals and what little bicarbonates/carbonates that are available (which is where a Calcium Reactor MAY be helpful in marine aquariums). For this reason, the use of crushed coral is more effective in a “Filter Bag” to release these minerals when used in Freshwater, especially African Cichlid aquaria. The use of a filter bag in a high flow area will improve the dissolution rate releasing more minerals and allowing for some buffering (as well as slightly improved adding mineral cations), although again I will point out not much carbonate (KH) buffering due to the mineral make up of crushed coral (Aragonite will do a slightly better buffering job when employed in a filter bag).
This said, despite the popular use of crushed coral/Aragonite for pH/KH control in African Cichlids, it is a poor choice for this, especially in high bio load aquariums due to the FACT of its mineral make up.
Simply put, you CANNOT make a mineral appear out of nowhere that does not exist and that seems to be what many mistakenly believe when using crushed coral to increase KH/pH. This is an unfortunate “aquatic myth” that many forums still perpetuate when a quick search of the chemical makeup of crushed coral would expose this folly.
And for those who use this equation to state otherwise: H2O + CO2 + CaCO3 <> Ca + 2HCO3, let me point out that this is great when used in a reaction chamber, but in the real world of an aquarium, I've yet to find that provides much buffering, including in marine aquariums with copious amounts of crushed coral. I still had to add additional buffers! Sorry actual experience with 1000s of aquariums trumps here!
The bottom line is that Crushed Coral MAY help maintain KH/pH in a low bio load aquarium, they are best used for minerals (GH). Even then, Wonder Shells (or similar mineral ion supplements such as AAP/SeaChem Replenish or Fresh Trace used regularly or in drip) are far superior to Crushed Coral due to the fact a Wonder Shell dissolves at a much faster rate and reacts much quicker to chemistry changes in the water than does crushed coral. As a well the use of a slow drip liquid mineral replenisher would also be superior to the use of Crushed Coral for mineral depletion. Besides the simple mineral makeup of crushed coral, my own extensive tests show that its use to increase KH as well as GH (Calcium and other minerals) simply is poor.
Product Resource:
• AAP Wonder Shells
• SeaChem Replenish; Can be added directly or by drip
Reference:
• The Mineral Argonite
In lower pH community tanks (6.8 to 7.4) KH (Alkaline) buffers such as Sea Chem Alkaline Buffer are still important, however, as noted earlier, I like to counter these with natural lower (acid) pH “buffers” such as Indian Almond Leaves, Peat, Pillow/Frog Moss, and/or Mango/Driftwood.
Buffering your freshwater aquarium is especially important if you have plants fed by CO2 which will raise pH during peak growth times, and there is scientific evidence that GH plays a role here as well.
Please see this article for more about this subject:
• AQUARIUM PLANTS; PROPER NUTRIENTS
For low pH/Soft Water Aquariums (such as Discus, Ram Cichlid, Betta), besides the before mentioned “slow” acid buffers (peat, Frog Moss, etc.), you can use immediate acid buffers with KH (carbonate) buffers for quicker results, such as SeaChem’s Acid Buffer.
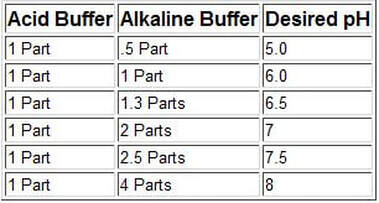
This is especially important when the use of 100% Reverse Osmosis water is employed: An Acid Buffer should be combined with an Alkaline Buffer in the ratios outlined in the chart to the left (this chart is for use with 100% RO or DI water ONLY).
Please note that these ratios are not hard and fast rules as each aquarium is very unique; so, testing is 100% required and even then, testing should be performed hours or even a day later to allow the chemistry to "settle". Once you establish a "sweet spot" for your unique aquarium environment, use the ratio numbers that work for you!
It is also noteworthy that these buffers only address pH and carbonate hardness and not essential mineral cations. So please read this article for a more in-depth explanation of the use of RO or DI water for aquarium use:
• Use of RO/DI Water in Aquarium
Back to baking soda: This is an old standby method based on the fact that baking soda does raise pH and KH, the problem is there is much new research to show that calcium, magnesium, electrolytes and Redox play a more important role in aquatic chemistry than just pH or basic KH alone (which is another reason 'good old fashioned water changes' often help improve fish health). If Baking Soda is used, I recommend using Wonder Shells or similar products (such as AAP/SeaChem Replenish or Aragamix) to added needed calcium and other important electrolytes.
Product Resources:
• FRESH AAP Wonder Shells; Including the Unique version sold NOWHERE else but at AAP
• AAP/SeaChem Replenish; for both soft and hard mineral depletion
If you have a very unstable KH level (drops rapidly), look into causes such as a large amount of decomposing organic material. The more organic break down (de-nitrification), the more acids produced. Some filters if not cleaned regularly can cause this, including canister, UGF, and Wet/Dry.
Please read the sections about too low pH and KH problems, as well as the Amazon River section lower in this article for more about solutions/causes to an unstable KH. This is especially important if you are considering using products such as pH Down or pH Up (which should never be used by an aquarium keeper that desires healthy & balanced aquarium chemistry).
For a really informative (and scientific) article about the relation of KH (Buffers) and pH, please follow this link:
• pH of buffer - Henderson-Hasselbalch equation
Another interesting scientific thread dealing with KH:
• The Carbonate Buffer
Or this excellent article:
• SeaChem's The Art and Science of Aquarium Management
Please note that these ratios are not hard and fast rules as each aquarium is very unique; so, testing is 100% required and even then, testing should be performed hours or even a day later to allow the chemistry to "settle". Once you establish a "sweet spot" for your unique aquarium environment, use the ratio numbers that work for you!
It is also noteworthy that these buffers only address pH and carbonate hardness and not essential mineral cations. So please read this article for a more in-depth explanation of the use of RO or DI water for aquarium use:
• Use of RO/DI Water in Aquarium
Back to baking soda: This is an old standby method based on the fact that baking soda does raise pH and KH, the problem is there is much new research to show that calcium, magnesium, electrolytes and Redox play a more important role in aquatic chemistry than just pH or basic KH alone (which is another reason 'good old fashioned water changes' often help improve fish health). If Baking Soda is used, I recommend using Wonder Shells or similar products (such as AAP/SeaChem Replenish or Aragamix) to added needed calcium and other important electrolytes.
Product Resources:
• FRESH AAP Wonder Shells; Including the Unique version sold NOWHERE else but at AAP
• AAP/SeaChem Replenish; for both soft and hard mineral depletion
If you have a very unstable KH level (drops rapidly), look into causes such as a large amount of decomposing organic material. The more organic break down (de-nitrification), the more acids produced. Some filters if not cleaned regularly can cause this, including canister, UGF, and Wet/Dry.
Please read the sections about too low pH and KH problems, as well as the Amazon River section lower in this article for more about solutions/causes to an unstable KH. This is especially important if you are considering using products such as pH Down or pH Up (which should never be used by an aquarium keeper that desires healthy & balanced aquarium chemistry).
For a really informative (and scientific) article about the relation of KH (Buffers) and pH, please follow this link:
• pH of buffer - Henderson-Hasselbalch equation
Another interesting scientific thread dealing with KH:
• The Carbonate Buffer
Or this excellent article:
• SeaChem's The Art and Science of Aquarium Management
GH & TDS
The section following this section deals with Calcium specifically.
General hardness (GH or dGH) refers to the dissolved concentration primarily of calcium, magnesium and other mineral ions. Both Calcium and magnesium are important for proper internal osmotic processes in fish (and invertebrates). More specifically, 1 dGH is defined as 10 milligrams (mg) of calcium oxide (CaO) per liter of water. Since CaO has a molar mass of 56.08 g/mol, 1 dGH is equivalent to 0.17832 mmol per litre of elemental calcium and/or magnesium ions.
Reference:
• Wikipedia; dGH
Other ions can contribute to water hardness but are usually insignificant and difficult to measure. When fish are said to prefer “soft” or “hard” water, it is GH, not the KH that is being referred to. GH will generally not directly affect pH although "hard" water is generally alkaline due to some interaction of GH and KH. It should also be noted that GH DOES effect pH when photosynthesis is thrown into the equation, please see this article: Planted Freshwater Aquariums: Nutrients & GH during photosynthesis.
TDS: Before I delve too much into GH, let me touch on the term "TDS" which is simply "Total Dissolved Solids". TDS measures conductivity and pretty much everything that is in the water. In other words, TDS is correlated to GH and frankly everything else in water that can be a solid. TDS is what you get if you let all the water evaporate out of a container. Total undissolved Solids. A healthy TDS reading in a planted freshwater and most community freshwater would be about 300 ppm, while an East African Cichlid tank would be closer to 800 ppm.
Basically, you can have a high TDS, but low GH, which is why that although a TDS meter is a good device to have for any advanced aquarium keeper, it still does not take the place of a GH & KH test kit. As well, using only a TDS meter can result in potentially a poor RH score (part of Redox Balance) since it measures overall conductivity, but this could possibly exclude essential calcium Cations needed for healthy Redox reduction. One way to look at a TDS meter is as an ongoing gauge of basic minerals, but just like a test strip, any subtle changes should be looked at by an actual specific water test.
Referenced Reading/Discussion:
• Understanding GH & TDS & Hardness
Product Resource:
• AAP TDS Monitor/Meter
Back to GH in general: Although many aquarists worry about “too high GH” (based on respiration problems), this is based on long ago proven false myths. In reality, freshwater generally would take a GH of over 500 ppm to cause this problem. This said, keeping a GH closer to the fish' natural biotope is certainly desirable, if only for the fact some diseases such as Columnaris can adhere better at high GH levels (especially above 400 pm), Just do not make the mistake of driving out mineral Cations in the process which could lower natural resistance to diseases.
More importantly as to respiration, the surface epithelia of gills and body surfaces are protected from direct interaction with the environment by mucous and intercellular junctions. Fish mucous has been postulated to have calcium binding properties. Mucous is a glycoprotein and could serve as a calcium chelating agent retarding ion loss from epithelial cells as a charged surface coat or barrier and thus is dependent on calcium for normal function. Intercellular junctions are specialized areas of attachment between epithelial cells preventing the loss of ions and fluids from the membrane which bathes and surrounds the cells beneath.
Reference:
• MEMBRANE PERMEABILITY, CALCIUM, AND OSMOTIC PRESSURE
In reality when GH is truly "too high" one is providing the opposite positive effect on respiration whereby we are providing too much Redox Reduction thus blocking adequate respiration. In other words, we need to understand more about aquarium Redox balance whereby too much oxidation or too much reduction can be a problem. Unfortunately, most aquarium keepers tend to err on the side of too much oxidation and shortchange their aquarium inhabitants on the importance of reduction via mineral Cations.
GH is an area of aquarium chemistry where there is a lot of misunderstanding or simply downright wrong advice. I have received many calls, emails, etc. over the years asking what do about their so-called high GH. Often this GH turns out to be only around 200-300 ppm which is fine for most fish (low for African Cichlids, livebearers and even goldfish do better at higher GH).
This concern is based on old assumptions of respiration in fish, as well as lack of understanding of the importance of positive Calcium ions (as well as Magnesium & Potassium) in the regulation of MANY bio processes in fish including healing, heart function, and regulation of osmotic functions.
As noted earlier, GH even plays a role in pH control in planted aquariums. Similar processes are also at work in marine aquariums, which is why the popularity of Kalkwasser, although GH is not referred to in Marine Aquariums, rather separate Calcium and other tests are performed. I recommend reading the section later in this article; “Calcium Carbonate”, which explains more about Calcium as well as many of the outside links/references.
This misunderstanding of GH also flies in the face of the best research to date about the importance of Redox in aquarium health and since Calcium and Magnesium play a role in a balanced Redox (as can UVC Sterilization), understanding that you may have a high GH, YET your aquariums Calcium or other Redox reducers may have given up all possible positive charges (cations) to cells (or other bio processes) under oxidation. It is for this reason, then, that calcium and magnesium supplies MUST be constantly renewed; without this “fresh” calcium, etc. your Redox balance and of course fish (or other aquatic inhabitants such as coral, frogs, shrimp) will suffer!
Important References:
• Aquarium Redox Balance; Including Corrected Ionization of Minerals
• UVC Sterilization
It is also noteworthy that a GH test is not always an accurate measure of positive calcium and other mineral ions (cations), as with many Ammonia test kits, which are inaccurate after using products such as Prime and give false positive for ammonia NH3 as they cannot discern the difference between the NH3 and NH4+.
The same can be said for GH tests that may show a high GH (despite the fact these test kits test for free divalent cations), when in reality all positive calcium ions are depleted due to Redox balancing, which is why one MUST constantly supply these mineral cations by whatever means, whether by regular water changes (which often are not enough, especially in small aquariums) or by use of mineral replenishers such as Wonder Shells (which will throw off accurate readings of GH test kits due to their constant supply of positive mineral ions such as calcium!!!).
General hardness (GH or dGH) refers to the dissolved concentration primarily of calcium, magnesium and other mineral ions. Both Calcium and magnesium are important for proper internal osmotic processes in fish (and invertebrates). More specifically, 1 dGH is defined as 10 milligrams (mg) of calcium oxide (CaO) per liter of water. Since CaO has a molar mass of 56.08 g/mol, 1 dGH is equivalent to 0.17832 mmol per litre of elemental calcium and/or magnesium ions.
Reference:
• Wikipedia; dGH
Other ions can contribute to water hardness but are usually insignificant and difficult to measure. When fish are said to prefer “soft” or “hard” water, it is GH, not the KH that is being referred to. GH will generally not directly affect pH although "hard" water is generally alkaline due to some interaction of GH and KH. It should also be noted that GH DOES effect pH when photosynthesis is thrown into the equation, please see this article: Planted Freshwater Aquariums: Nutrients & GH during photosynthesis.
TDS: Before I delve too much into GH, let me touch on the term "TDS" which is simply "Total Dissolved Solids". TDS measures conductivity and pretty much everything that is in the water. In other words, TDS is correlated to GH and frankly everything else in water that can be a solid. TDS is what you get if you let all the water evaporate out of a container. Total undissolved Solids. A healthy TDS reading in a planted freshwater and most community freshwater would be about 300 ppm, while an East African Cichlid tank would be closer to 800 ppm.
Basically, you can have a high TDS, but low GH, which is why that although a TDS meter is a good device to have for any advanced aquarium keeper, it still does not take the place of a GH & KH test kit. As well, using only a TDS meter can result in potentially a poor RH score (part of Redox Balance) since it measures overall conductivity, but this could possibly exclude essential calcium Cations needed for healthy Redox reduction. One way to look at a TDS meter is as an ongoing gauge of basic minerals, but just like a test strip, any subtle changes should be looked at by an actual specific water test.
Referenced Reading/Discussion:
• Understanding GH & TDS & Hardness
Product Resource:
• AAP TDS Monitor/Meter
Back to GH in general: Although many aquarists worry about “too high GH” (based on respiration problems), this is based on long ago proven false myths. In reality, freshwater generally would take a GH of over 500 ppm to cause this problem. This said, keeping a GH closer to the fish' natural biotope is certainly desirable, if only for the fact some diseases such as Columnaris can adhere better at high GH levels (especially above 400 pm), Just do not make the mistake of driving out mineral Cations in the process which could lower natural resistance to diseases.
More importantly as to respiration, the surface epithelia of gills and body surfaces are protected from direct interaction with the environment by mucous and intercellular junctions. Fish mucous has been postulated to have calcium binding properties. Mucous is a glycoprotein and could serve as a calcium chelating agent retarding ion loss from epithelial cells as a charged surface coat or barrier and thus is dependent on calcium for normal function. Intercellular junctions are specialized areas of attachment between epithelial cells preventing the loss of ions and fluids from the membrane which bathes and surrounds the cells beneath.
Reference:
• MEMBRANE PERMEABILITY, CALCIUM, AND OSMOTIC PRESSURE
In reality when GH is truly "too high" one is providing the opposite positive effect on respiration whereby we are providing too much Redox Reduction thus blocking adequate respiration. In other words, we need to understand more about aquarium Redox balance whereby too much oxidation or too much reduction can be a problem. Unfortunately, most aquarium keepers tend to err on the side of too much oxidation and shortchange their aquarium inhabitants on the importance of reduction via mineral Cations.
GH is an area of aquarium chemistry where there is a lot of misunderstanding or simply downright wrong advice. I have received many calls, emails, etc. over the years asking what do about their so-called high GH. Often this GH turns out to be only around 200-300 ppm which is fine for most fish (low for African Cichlids, livebearers and even goldfish do better at higher GH).
This concern is based on old assumptions of respiration in fish, as well as lack of understanding of the importance of positive Calcium ions (as well as Magnesium & Potassium) in the regulation of MANY bio processes in fish including healing, heart function, and regulation of osmotic functions.
As noted earlier, GH even plays a role in pH control in planted aquariums. Similar processes are also at work in marine aquariums, which is why the popularity of Kalkwasser, although GH is not referred to in Marine Aquariums, rather separate Calcium and other tests are performed. I recommend reading the section later in this article; “Calcium Carbonate”, which explains more about Calcium as well as many of the outside links/references.
This misunderstanding of GH also flies in the face of the best research to date about the importance of Redox in aquarium health and since Calcium and Magnesium play a role in a balanced Redox (as can UVC Sterilization), understanding that you may have a high GH, YET your aquariums Calcium or other Redox reducers may have given up all possible positive charges (cations) to cells (or other bio processes) under oxidation. It is for this reason, then, that calcium and magnesium supplies MUST be constantly renewed; without this “fresh” calcium, etc. your Redox balance and of course fish (or other aquatic inhabitants such as coral, frogs, shrimp) will suffer!
Important References:
• Aquarium Redox Balance; Including Corrected Ionization of Minerals
• UVC Sterilization
It is also noteworthy that a GH test is not always an accurate measure of positive calcium and other mineral ions (cations), as with many Ammonia test kits, which are inaccurate after using products such as Prime and give false positive for ammonia NH3 as they cannot discern the difference between the NH3 and NH4+.
The same can be said for GH tests that may show a high GH (despite the fact these test kits test for free divalent cations), when in reality all positive calcium ions are depleted due to Redox balancing, which is why one MUST constantly supply these mineral cations by whatever means, whether by regular water changes (which often are not enough, especially in small aquariums) or by use of mineral replenishers such as Wonder Shells (which will throw off accurate readings of GH test kits due to their constant supply of positive mineral ions such as calcium!!!).
Depletion of Positive Ions
My ORP readings, experience, and visual tests confirm this too as the picture to the right clearly demonstrates where by potasium permanganate (an oxidizer) was added in equal doses with one using water changes and the other a Regular AAP Wonder Shell. It is noteworthy that the GH statistically was the same between the aquariums with clean water and the one with the AAP Wonder Shell.
Examples of GH and Important mineral Ions in an Aquarium/Pond:
• Depletion of Positive Ions: Think of it this way; a storage battery "works" only when a positive and a negative electrode are present to maintain an electrical current. When the positive ion charged plates become exhausted, the battery is no longer any good until recharged. In lead/acid batteries essentially lead (Pb) and lead oxide (PbO2) are changed to lead (II) sulfate (PbSO) in the discharged state (exhausted positive ions), HOWEVER the lead is still present (as with calcium in an aquarium) in a discharged state.
Water changes and additional supplements are generally necessary to replenish these mineral ions (cations). Using the car battery as an example again, when re-charging, a 12 volt battery will show a charge of up to 14 volts in a 12 volt battery shortly after and at the completion of a charge, this is similar to the high GH (often over 400) with the use of many methods of adding mineral electrolytes such as genuine AAP Wonder Shells.
Also beware that not all calcium is the same for adding positive ions (Cations); you want to use a bio-available calcium. Calcium Carbonate can provide mineral Cations when charged. Calcium Sulfate and Calcium Chloride generally do not because of the usual negative charge (Anions) in Chloride and Sulfates.
Reference:
• Cation/ Anion List
Examples of GH and Important mineral Ions in an Aquarium/Pond:
• Depletion of Positive Ions: Think of it this way; a storage battery "works" only when a positive and a negative electrode are present to maintain an electrical current. When the positive ion charged plates become exhausted, the battery is no longer any good until recharged. In lead/acid batteries essentially lead (Pb) and lead oxide (PbO2) are changed to lead (II) sulfate (PbSO) in the discharged state (exhausted positive ions), HOWEVER the lead is still present (as with calcium in an aquarium) in a discharged state.
Water changes and additional supplements are generally necessary to replenish these mineral ions (cations). Using the car battery as an example again, when re-charging, a 12 volt battery will show a charge of up to 14 volts in a 12 volt battery shortly after and at the completion of a charge, this is similar to the high GH (often over 400) with the use of many methods of adding mineral electrolytes such as genuine AAP Wonder Shells.
Also beware that not all calcium is the same for adding positive ions (Cations); you want to use a bio-available calcium. Calcium Carbonate can provide mineral Cations when charged. Calcium Sulfate and Calcium Chloride generally do not because of the usual negative charge (Anions) in Chloride and Sulfates.
Reference:
• Cation/ Anion List
The picture above is of a Redox Meter that can be used to test before and after using water conditioners, addition of positive mineral ion calcium such as AAP Wonder Shells.
Resource:
• EcoSense ORP 15 Redox Meter
Increasing GH Readings as per Tests: Often when an aquarium keeper only “tops off” for evaporation or performs inadequate/small water changes the GH may actually climb in areas of hard water. This does not indicate a healthy aquarium, rather quite the opposite as the small amount of mineral ions that are added via “top offs” or small water changes will not keep up with depletion even though your GH test shows a climbing GH (General Hardness). Using the auto/RV battery example again; simply throwing more lead into your battery may increase lead content but does not necessarily increase the electrical charge.
The bottom line is adequate water changes (but not necessarily large in a mature/healthy aquarium) often along with mineral supplements are required in a closed aquarium/pond to keep healthy ionization in your aquarium/pond and a stable GH is an indicator of this, not a climbing GH (although many supplements will increase GH until water changes).
This is where I respectfully disagree with those promoting the "Walstad" method and recommendations to aim for unreasonable GH levels in many aquarium biotopes. Instead, I refer to Atison Phumchoosri or Marc Weiss as per recommendations of GH & calcium (besides my own VERY DEPTH tests in closed aquarium systems). For much more, see the "Providing Amazon/SE Asia River/West Africa Water" of this article.
See this article for more on this subject:
• Redox Balance in Aquariums; Importance of reduction in fish health
Product Resources:
• Wonder Shells for Positive Mineral Ions; Including the Unique version sold NOWHERE else but at AAP
Resource:
• EcoSense ORP 15 Redox Meter
Increasing GH Readings as per Tests: Often when an aquarium keeper only “tops off” for evaporation or performs inadequate/small water changes the GH may actually climb in areas of hard water. This does not indicate a healthy aquarium, rather quite the opposite as the small amount of mineral ions that are added via “top offs” or small water changes will not keep up with depletion even though your GH test shows a climbing GH (General Hardness). Using the auto/RV battery example again; simply throwing more lead into your battery may increase lead content but does not necessarily increase the electrical charge.
The bottom line is adequate water changes (but not necessarily large in a mature/healthy aquarium) often along with mineral supplements are required in a closed aquarium/pond to keep healthy ionization in your aquarium/pond and a stable GH is an indicator of this, not a climbing GH (although many supplements will increase GH until water changes).
This is where I respectfully disagree with those promoting the "Walstad" method and recommendations to aim for unreasonable GH levels in many aquarium biotopes. Instead, I refer to Atison Phumchoosri or Marc Weiss as per recommendations of GH & calcium (besides my own VERY DEPTH tests in closed aquarium systems). For much more, see the "Providing Amazon/SE Asia River/West Africa Water" of this article.
See this article for more on this subject:
• Redox Balance in Aquariums; Importance of reduction in fish health
Product Resources:
• Wonder Shells for Positive Mineral Ions; Including the Unique version sold NOWHERE else but at AAP
Further GH Suggestions: Products such as Wonder Shells, similar mineral blocks, or powders such as AragaMight are excellent for mineral cation (GH) maintenance. As well the use of aragonite in filters can also be employed for mineral cation maintenance although my experience has shown the method of using aragonite to be less responsive to rapid changes in positive mineral ion needs in FW (especially planted aquariums) than the use of Mineral Blocks or drip methods.
Mineral Blocks such as Wonder Shells that dissolve slowly and thus keep a more balanced positive mineral ion level are the best method from my experience, however powders such as AragaMight (by CaribSea) can be pre-dissolved and then dripped slowly from 2 liter bottle (or similar) using airline tubing and an airline control valve to aid in a slow drip are a good alternative (more so for larger tanks as most drip rates would be too rapid for small tanks).
As noted earlier, another product that can be used for this process is SeaChem Replenish (or AAP/SeaChem Fresh Trace or Equilibrium) mixed into a volume of water and then dripped into the aquarium (this is an excellent product for Amazon River biotope tanks). However, these products, based on my experimentation/use are meant to be for entire water re-mineralization use with RO/DI water or adding to water after a water change with water that is poorly mineralized at its source. The Wonder Shells still do a better job with providing these essential mineral Cations, even if just fragments are used (which is all that is really necessary for fish such as Discus or Ram Cichlids, not the full dosage recommended by some sellers such as Amazon that know nothing about this product and do not even sell all types of these mineral blocks).
SeaChem Cichlid Salt also has essential minerals along with salt (sodium chloride) in the CORRECT ionic ratios based on the amount of salt one would add to most freshwater applications. HOWEVER, products such as API Aquarium Salt only has ratios that would require enough salt added to nearly a saltwater aquarium to achieve essential minerals for most freshwater applications (especially cichlids and livebearers), making this product (API Aquarium Salt) an extremely poor choice for mineral replenishment.
Finally, an obvious way to replace minerals (electrolytes/cations) is also via regular water changes (with water that contains minerals, not un-replenished RO Water). It is noteworthy that with high bio load aquariums or high mineral need aquariums such as Molly or other livebearer tanks, water changes are often not adequate. As my tests (especially those conducted in the 1990s) show that a constant supply of these mineral improved disease resistance.
See:
• A Healthy Aquarium; Disease Prevention
It should also be noted that your GH may be artificially high from the use of mineral blocks/powders, however much of this is Calcium of which most all fish (fresh and saltwater as well as invertebrates) can tolerate in high levels.
Mineral Blocks such as Wonder Shells that dissolve slowly and thus keep a more balanced positive mineral ion level are the best method from my experience, however powders such as AragaMight (by CaribSea) can be pre-dissolved and then dripped slowly from 2 liter bottle (or similar) using airline tubing and an airline control valve to aid in a slow drip are a good alternative (more so for larger tanks as most drip rates would be too rapid for small tanks).
As noted earlier, another product that can be used for this process is SeaChem Replenish (or AAP/SeaChem Fresh Trace or Equilibrium) mixed into a volume of water and then dripped into the aquarium (this is an excellent product for Amazon River biotope tanks). However, these products, based on my experimentation/use are meant to be for entire water re-mineralization use with RO/DI water or adding to water after a water change with water that is poorly mineralized at its source. The Wonder Shells still do a better job with providing these essential mineral Cations, even if just fragments are used (which is all that is really necessary for fish such as Discus or Ram Cichlids, not the full dosage recommended by some sellers such as Amazon that know nothing about this product and do not even sell all types of these mineral blocks).
SeaChem Cichlid Salt also has essential minerals along with salt (sodium chloride) in the CORRECT ionic ratios based on the amount of salt one would add to most freshwater applications. HOWEVER, products such as API Aquarium Salt only has ratios that would require enough salt added to nearly a saltwater aquarium to achieve essential minerals for most freshwater applications (especially cichlids and livebearers), making this product (API Aquarium Salt) an extremely poor choice for mineral replenishment.
Finally, an obvious way to replace minerals (electrolytes/cations) is also via regular water changes (with water that contains minerals, not un-replenished RO Water). It is noteworthy that with high bio load aquariums or high mineral need aquariums such as Molly or other livebearer tanks, water changes are often not adequate. As my tests (especially those conducted in the 1990s) show that a constant supply of these mineral improved disease resistance.
See:
• A Healthy Aquarium; Disease Prevention
It should also be noted that your GH may be artificially high from the use of mineral blocks/powders, however much of this is Calcium of which most all fish (fresh and saltwater as well as invertebrates) can tolerate in high levels.
Magnesium
Magnesium is important for proper osmotic functions in fish and invertebrates. Magnesium is essential for Calcium assimilation, so when magnesium levels are low, the calcium supply becomes exhausted. For this reason, Magnesium is better added in the proper balance with calcium (which both are essential to each other for proper utilization), in such products as Wonder Shells or aragonite sand in a small bag in the filter (although the latter is not as reliable). Magnesium should be part of your overall mineral balance in your aquarium and kept at a level of 1200 to 1400 ppm in marine aquariums (lower for freshwater).
Epsom salts that contain magnesium sulfate, are best used for therapeutic reasons such as to aid in flushing the system as it aids in and speeds osmotic function and helps to move fluids out of the body.
Sulfates, one of the major components of Epsom Salt, have been shown effective in improving nutrient absorption and toxin elimination.
Magnesium, the other major component of Epsom Salt, plays a role in the activity of more than 325 enzymes. That said you would only want to add about 1/4- 1/2 teaspoon per 5 gallons (18 liters). Although useful for therapeutic reasons, magnesium and sulfates in particular are best introduced as part of a greater trace element balance in an aquarium. I ONLY recommend Epsom Salts for therapeutic aquarium treatment, NOT on an ongoing basis. The best use is 1/4- 1/2 teaspoon per 1 gallon of a 30-minute Fish Bath.
Also note that Epsom salts (as well as sodium chloride; regular table salt) do NOT evaporate or decompose like antibiotics, so only add more after water changes.
• NOTE: Ongoing use of Epsom Salt in the display aquarium can have opposite effect of causing osmoregulation issues, as well as slowed heartbeat and possibly eventual death. What is noteworthy is that Magnesium is not normally depleted at very high rates (even in marine aquariums I found the need to add additional Magnesium very infrequent). QUOTE from article below: "As for magnesium sulfate (Epsom salt), while I do not specifically know the mechanism, what I do know from experimenting around is that it works well in a bath but is actually detrimental “in-tank” for freshwater. My guess is magnesium is more of a controlling osmoregulator like sodium chloride can be as well, not something the fish need much of. As an analogy, think of how our atmosphere is mostly nitrogen, yet it is oxygen that we utilize. Ditto magnesium sulfate in water, especially marine aquariums, but over loading in freshwater long term seems to have a negative effect, while a short-term bath of magnesium sulfate seems to help draw fluids though the fish in a therapeutic way."
Reference:
• Fish Baths, Dips, Swabs; Including Coral; For Disease, Ammonia, Treatment
Other needs for Magnesium:
• Normal calcium balance in organs.
• Healthy muscles.
• Healthy nerve transduction.
• Healthy calcium balance in blood vessels.
Further References:
• Osmosis in Fish, including Magnesium
• Fish Baths
• The Magnesium Web Site
Epsom salts that contain magnesium sulfate, are best used for therapeutic reasons such as to aid in flushing the system as it aids in and speeds osmotic function and helps to move fluids out of the body.
Sulfates, one of the major components of Epsom Salt, have been shown effective in improving nutrient absorption and toxin elimination.
Magnesium, the other major component of Epsom Salt, plays a role in the activity of more than 325 enzymes. That said you would only want to add about 1/4- 1/2 teaspoon per 5 gallons (18 liters). Although useful for therapeutic reasons, magnesium and sulfates in particular are best introduced as part of a greater trace element balance in an aquarium. I ONLY recommend Epsom Salts for therapeutic aquarium treatment, NOT on an ongoing basis. The best use is 1/4- 1/2 teaspoon per 1 gallon of a 30-minute Fish Bath.
Also note that Epsom salts (as well as sodium chloride; regular table salt) do NOT evaporate or decompose like antibiotics, so only add more after water changes.
• NOTE: Ongoing use of Epsom Salt in the display aquarium can have opposite effect of causing osmoregulation issues, as well as slowed heartbeat and possibly eventual death. What is noteworthy is that Magnesium is not normally depleted at very high rates (even in marine aquariums I found the need to add additional Magnesium very infrequent). QUOTE from article below: "As for magnesium sulfate (Epsom salt), while I do not specifically know the mechanism, what I do know from experimenting around is that it works well in a bath but is actually detrimental “in-tank” for freshwater. My guess is magnesium is more of a controlling osmoregulator like sodium chloride can be as well, not something the fish need much of. As an analogy, think of how our atmosphere is mostly nitrogen, yet it is oxygen that we utilize. Ditto magnesium sulfate in water, especially marine aquariums, but over loading in freshwater long term seems to have a negative effect, while a short-term bath of magnesium sulfate seems to help draw fluids though the fish in a therapeutic way."
Reference:
• Fish Baths, Dips, Swabs; Including Coral; For Disease, Ammonia, Treatment
Other needs for Magnesium:
• Normal calcium balance in organs.
• Healthy muscles.
• Healthy nerve transduction.
• Healthy calcium balance in blood vessels.
Further References:
• Osmosis in Fish, including Magnesium
• Fish Baths
• The Magnesium Web Site
Calcium
Calcium carbonate in your aquarium will keep a more stable electrolyte balance (for osmotic function), while magnesium is another important element that works with calcium. A proper amount of Calcium and Magnesium in your aquarium will affect the fish health positively, including fish such as Discus, German Rams and Apistos. See the Amazon River Section for more as to the use of calcium with soft water fish!
Magnesium and calcium have been shown to increase resistance to degenerate diseases by lowering the acidity in the body. This will help with prevention of ich, fungus, and general “wear and tear” in your fish.
Calcium also helps in healing and stress, and without proper calcium levels healing may be difficult or impossible.
Calcium is also important and has been shown to both prevent and at least in part treat 'Hole in the Head' disease common to cichlids (also referred to as HITH). It is noteworthy that the addition of antibiotics (such as Tetracycline) will lower calcium absorption, while the presence of correct amounts of calcium in the aquarium water will considerably reduce the toxic side effects of Malachite Green which is why a GH (for freshwater calcium measurement) of at least 100 ppm or higher is SO VERY important to Ich treatment.
Further References:
• Hole in the Head Disease (HITH)
• Aquarium Medications Chemical Treatments; Malachite Green
• Aquarium Ich Treatment, Prevention, more
In fact, I will go a step further as need for Calcium (even in freshwater) seems to be totally misunderstood. I have observed this quite a lot in both scientific and non-scientific observations over the years.
Here is an important quote: “The presence of free (ionic) calcium at relatively high concentrations in culture water helps reduce the loss of other salts (e.g., sodium and potassium) from fish body fluids (i.e., blood). Sodium and potassium are the most important salts in fish blood and are critical for normal heart, nerve and muscle function. In low calcium water, fish can lose (leak) substantial quantities of these salts into the water.”
In freshwater aquariums I recommend a minimum of 100 ppm of calcium (which a GH of 100+ ppm will generally provide).
Please also read this article from Aquarium Answers as well, as much of what is contained in this article relates to the importance of Calcium and other minerals as well:
• Proper Osmotic function, Do Fish Drink
Another note about calcium; Calcium is very important to proper discus health, yet calcium can adversely affect the kH of a discus aquarium when combined with sodium carbonates or bi carbonates, which is generally kept at a pH below 6.5. I have successfully used sources of calcium such as Wonder Shells (usually at 1/4 -1/2 dose), in discus aquariums by using a mix of RO (Reverse Osmosis) water and tap water (dilution will vary depending on your tap and tank water parameters). I then add electrolytes to the RO water and add peat to the filters. I have used this method successfully with discus and added the needed calcium with no pH climb.
Calcium is also a major Reducer with a negative 2.87 electron reduction rating, and plays an important role in maintaining a healthy aquarium Redox Balance that is a VERY important aspect of true water quality.
Reference:
• Aquarium Redox Balance, ORP
Finally, back to the aspect of Calcium (& magnesium) in general is if these important elements are in “short supply” besides the before mentioned problems, an aquarist may also see pH swings a few hours after the lights go on as the process of Photosynthesis in algae will increase pH during daylight hours if low levels of calcium and magnesium are present (as noted earlier in the GH section as per studies).
Keep in mind that a pH swing from 7.8 to 8.2 (as is common in this situation) is four fold increase in pH since the pH scale is logarithmic.
Other needs for calcium:
• Calcium is a vital component in blood clotting systems and also helps in wound healing.
• Calcium helps to control nerve transmission, and release of neurotransmitters.
• Calcium is an essential component in the production of enzymes and hormones that regulate digestion, energy, and fat metabolism.
• Calcium helps to transport ions (electrically charged particles) across the membrane.
• Calcium is essential for muscle contraction.
• Calcium assists in maintaining all cells and connective tissues in the body.
For further reading about Calcium and other minerals, please reference the articles below.
For relationship of mineral bioavailability when positive and negative ions are considered:
Soil Basics - How it Works
For more information about how Calcium, pH, GH, and KH relates to ponds, please see this article about Ponds:
• A Clear Pond; Pond Information
Magnesium and calcium have been shown to increase resistance to degenerate diseases by lowering the acidity in the body. This will help with prevention of ich, fungus, and general “wear and tear” in your fish.
Calcium also helps in healing and stress, and without proper calcium levels healing may be difficult or impossible.
Calcium is also important and has been shown to both prevent and at least in part treat 'Hole in the Head' disease common to cichlids (also referred to as HITH). It is noteworthy that the addition of antibiotics (such as Tetracycline) will lower calcium absorption, while the presence of correct amounts of calcium in the aquarium water will considerably reduce the toxic side effects of Malachite Green which is why a GH (for freshwater calcium measurement) of at least 100 ppm or higher is SO VERY important to Ich treatment.
Further References:
• Hole in the Head Disease (HITH)
• Aquarium Medications Chemical Treatments; Malachite Green
• Aquarium Ich Treatment, Prevention, more
In fact, I will go a step further as need for Calcium (even in freshwater) seems to be totally misunderstood. I have observed this quite a lot in both scientific and non-scientific observations over the years.
Here is an important quote: “The presence of free (ionic) calcium at relatively high concentrations in culture water helps reduce the loss of other salts (e.g., sodium and potassium) from fish body fluids (i.e., blood). Sodium and potassium are the most important salts in fish blood and are critical for normal heart, nerve and muscle function. In low calcium water, fish can lose (leak) substantial quantities of these salts into the water.”
In freshwater aquariums I recommend a minimum of 100 ppm of calcium (which a GH of 100+ ppm will generally provide).
Please also read this article from Aquarium Answers as well, as much of what is contained in this article relates to the importance of Calcium and other minerals as well:
• Proper Osmotic function, Do Fish Drink
Another note about calcium; Calcium is very important to proper discus health, yet calcium can adversely affect the kH of a discus aquarium when combined with sodium carbonates or bi carbonates, which is generally kept at a pH below 6.5. I have successfully used sources of calcium such as Wonder Shells (usually at 1/4 -1/2 dose), in discus aquariums by using a mix of RO (Reverse Osmosis) water and tap water (dilution will vary depending on your tap and tank water parameters). I then add electrolytes to the RO water and add peat to the filters. I have used this method successfully with discus and added the needed calcium with no pH climb.
Calcium is also a major Reducer with a negative 2.87 electron reduction rating, and plays an important role in maintaining a healthy aquarium Redox Balance that is a VERY important aspect of true water quality.
Reference:
• Aquarium Redox Balance, ORP
Finally, back to the aspect of Calcium (& magnesium) in general is if these important elements are in “short supply” besides the before mentioned problems, an aquarist may also see pH swings a few hours after the lights go on as the process of Photosynthesis in algae will increase pH during daylight hours if low levels of calcium and magnesium are present (as noted earlier in the GH section as per studies).
Keep in mind that a pH swing from 7.8 to 8.2 (as is common in this situation) is four fold increase in pH since the pH scale is logarithmic.
Other needs for calcium:
• Calcium is a vital component in blood clotting systems and also helps in wound healing.
• Calcium helps to control nerve transmission, and release of neurotransmitters.
• Calcium is an essential component in the production of enzymes and hormones that regulate digestion, energy, and fat metabolism.
• Calcium helps to transport ions (electrically charged particles) across the membrane.
• Calcium is essential for muscle contraction.
• Calcium assists in maintaining all cells and connective tissues in the body.
For further reading about Calcium and other minerals, please reference the articles below.
For relationship of mineral bioavailability when positive and negative ions are considered:
Soil Basics - How it Works
For more information about how Calcium, pH, GH, and KH relates to ponds, please see this article about Ponds:
• A Clear Pond; Pond Information
Kalkwass, Calcium Reactors, Balling
Your Calcium level should be 400-450 ppm for marine aquaria. There are many methods for calcium introduction in marine reef aquariums; from Calcium Chloride Dehydrate, oolitic sand (aragonite), Kalkwasser, Calcium reactors, and products such as "Reef Calcium; polygluconate", and SeaChem Reef Advantage Calcium (which is more than just calcium).
Product Resources:
• SeaChem Reef Calcium Bio-Available Polygluconate
• Reef Advantage Calcium from AAP
Although Kalkwasser is popular among many advanced marine aquarists, caution should be used with this method of adding calcium to your marine aquarium (this is not to say Kalkwasser does not work, in fact introduced properly it is an excellent way to regulate calcium and alkalinity in saltwater aquariums).
Here is how Kalkwasser works: Used properly Kalkwasser (Calcium Hydroxide) is slowly dripped into your aquarium, it captures free Carbon Dioxide present in the tank water and converts it to Bicarbonate ions. However, if you drip too fast or if there is not enough Carbon Dioxide available in the water, Carbonate ions will be formed which will make the Ca++ you are trying to add to your tank get wasted by the useless precipitation of Calcium Carbonate (often forming a white residue that precipitates out of your aquarium). Too rapid addition of Kalkwasser may actually cause the Calcium and Alkalinity in your tank to go down instead of up.
See the equation below:
• Ca++ + 2(HCO3-) + Ca++ + 2(OH-) <==> 2 CaCO3 + 2 H2O
Often even a slow drip of Kalkwasser (Calcium Hydroxide) can cause the above reaction if there is not enough CO2 present in your marine aquarium. One method to avoid this is to add 15ml of 5% Distilled White Vinegar (Acetic Acid) into a 1 liter or 1 quart container. Dissolve 1/2 teaspoon of Kalkwasser into the Acetic Acid, and then dilute to 1 liter (1 quart) volume with either RO/DI water (tank water can be used in a pinch).
Product Resources:
• SeaChem Reef Calcium Bio-Available Polygluconate
• Reef Advantage Calcium from AAP
Although Kalkwasser is popular among many advanced marine aquarists, caution should be used with this method of adding calcium to your marine aquarium (this is not to say Kalkwasser does not work, in fact introduced properly it is an excellent way to regulate calcium and alkalinity in saltwater aquariums).
Here is how Kalkwasser works: Used properly Kalkwasser (Calcium Hydroxide) is slowly dripped into your aquarium, it captures free Carbon Dioxide present in the tank water and converts it to Bicarbonate ions. However, if you drip too fast or if there is not enough Carbon Dioxide available in the water, Carbonate ions will be formed which will make the Ca++ you are trying to add to your tank get wasted by the useless precipitation of Calcium Carbonate (often forming a white residue that precipitates out of your aquarium). Too rapid addition of Kalkwasser may actually cause the Calcium and Alkalinity in your tank to go down instead of up.
See the equation below:
• Ca++ + 2(HCO3-) + Ca++ + 2(OH-) <==> 2 CaCO3 + 2 H2O
Often even a slow drip of Kalkwasser (Calcium Hydroxide) can cause the above reaction if there is not enough CO2 present in your marine aquarium. One method to avoid this is to add 15ml of 5% Distilled White Vinegar (Acetic Acid) into a 1 liter or 1 quart container. Dissolve 1/2 teaspoon of Kalkwasser into the Acetic Acid, and then dilute to 1 liter (1 quart) volume with either RO/DI water (tank water can be used in a pinch).
Calcium Reactors for Reef Tanks: As noted earlier in the Kalkwasser section, a Calcium Generator/Reactor is an excellent idea for maintenance of calcium and alkalinity as well as KH/pH regulation, especially in marine/reef tanks heavily stocked with stony corals. A calcium Reactor works by providing a steady stream of calcium by using CO2 in the reactor. The CO2 then "reacts" with the Calcium Carbonate producing calcium ions and carbonate ions (the carbonate ions increase alkalinity). Although I have NOT had an instance where this was an "essential" item for my reef tanks, it can be very useful for previously noted reasons.
Under normal circumstances/conditions a Fluidized Filter utilizing Oolitic sand media can accomplish similar results, however for high bio load reef tanks with large amounts of stony corals, a Calcium Reactor will produce more calcium.
The advantage in salt OR freshwater is similar to the simple Wonder Shells in freshwater (except better in my opinion); it provides an essential positive calcium ions, that are often exhausted, even when tests show calcium still present in the aquarium (See the section in red font in the GH section of this article).
Product Resource:
• Premium Fluidized Filters for Fresh or Saltwater Aquariums
Under normal circumstances/conditions a Fluidized Filter utilizing Oolitic sand media can accomplish similar results, however for high bio load reef tanks with large amounts of stony corals, a Calcium Reactor will produce more calcium.
The advantage in salt OR freshwater is similar to the simple Wonder Shells in freshwater (except better in my opinion); it provides an essential positive calcium ions, that are often exhausted, even when tests show calcium still present in the aquarium (See the section in red font in the GH section of this article).
Product Resource:
• Premium Fluidized Filters for Fresh or Saltwater Aquariums
Balling Method: An even newer method that is superior to Calcium Reactors for calcium supplementation/maintenance is the "Balling Method" developed by Hans-Werner Balling of Germany.
The Balling Method has become a popular method for dosing reef aquariums with essential elements, such as calcium, magnesium, among others, and maintaining adequate carbonate hardness alkalinity (carbonate hardness). This method is superior to Calcium Reactors as no acids nor phosphates are introduced to your marine/reef aquarium.
When used correctly, all levels of major elements remain constant, with calcium levels at 420 mg/l, magnesium at about 1300 mg/l and carbonate alkalinity at 7 dKH. Many German aquarists who have been using the Balling Method state this method accounted for a doubling in size of small-polyped stony (SPS) corals in their aquariums within about 100 days.
TMC (Tropic Marine Centre), a leader in German and British marine aquarium keeping, has a product that utilizes this "Balling" bio-calcium method in the 3 required steps.
For Marine fish or FOWLR aquariums, simply using the AAP SeaLab along with SeaChem/AAP Marine Buffer (for alkalinity maintenance as needed) is suggested for those wanting a more simple BUT PROVEN method for these more basic marine aquariums.
For more in depth discussion of Marine Fish & Reef Chemistry Maintenance, please read this excellent article:
• Reef Aquarium Chemistry Maintenance
Product Resources:
• TMC Bio Calcium for Balling Method from AAP
• AAP SeaLab
The Balling Method has become a popular method for dosing reef aquariums with essential elements, such as calcium, magnesium, among others, and maintaining adequate carbonate hardness alkalinity (carbonate hardness). This method is superior to Calcium Reactors as no acids nor phosphates are introduced to your marine/reef aquarium.
When used correctly, all levels of major elements remain constant, with calcium levels at 420 mg/l, magnesium at about 1300 mg/l and carbonate alkalinity at 7 dKH. Many German aquarists who have been using the Balling Method state this method accounted for a doubling in size of small-polyped stony (SPS) corals in their aquariums within about 100 days.
TMC (Tropic Marine Centre), a leader in German and British marine aquarium keeping, has a product that utilizes this "Balling" bio-calcium method in the 3 required steps.
For Marine fish or FOWLR aquariums, simply using the AAP SeaLab along with SeaChem/AAP Marine Buffer (for alkalinity maintenance as needed) is suggested for those wanting a more simple BUT PROVEN method for these more basic marine aquariums.
For more in depth discussion of Marine Fish & Reef Chemistry Maintenance, please read this excellent article:
• Reef Aquarium Chemistry Maintenance
Product Resources:
• TMC Bio Calcium for Balling Method from AAP
• AAP SeaLab
Plaster of Paris & Tums (and Lime)
Although long ago discredited, Plaster of Paris is often recommended as a source of calcium for ponds or aquariums in place of aragonite, Wonder Shells or similar. This is NOT a substitute for Wonder Shells aragonite or similar and is not the same!
Plaster of Paris is a poor choice for GH or Calcium as Calcium needs to be in the proper ratios with other elements such as Magnesium to be utilized by aquatic life (such as osmotic function). Lime is also similarly recommended for ponds for KH, GH and pH, however it is simply CaO and does not contain other elements necessary including bi-carbonates and magnesium to name but a few.
"Tums" are one more urban myth product recommended for GH & KH, but again this is only Calcium Carbonate and does not provide the completer minerals necessary for mineral ion replenishment nor ANY KH buffering!
Here is a list of ingredients of Plaster of Paris:
• CaSO4 , 1/2H2O: 97.97%
• SiO: 0.94%
• Al2O3: 0.69%
• Fe2O3: 0.28%
• MgO : 00.12%
For a further explanation of Plaster of Paris in aquariums and Ponds, please read this article:
• THE DIFFERENCE BETWEEN PLASTER OF PARIS AND PRODUCTS SUCH AS WONDER SHELLS
Plaster of Paris is a poor choice for GH or Calcium as Calcium needs to be in the proper ratios with other elements such as Magnesium to be utilized by aquatic life (such as osmotic function). Lime is also similarly recommended for ponds for KH, GH and pH, however it is simply CaO and does not contain other elements necessary including bi-carbonates and magnesium to name but a few.
"Tums" are one more urban myth product recommended for GH & KH, but again this is only Calcium Carbonate and does not provide the completer minerals necessary for mineral ion replenishment nor ANY KH buffering!
Here is a list of ingredients of Plaster of Paris:
• CaSO4 , 1/2H2O: 97.97%
• SiO: 0.94%
• Al2O3: 0.69%
• Fe2O3: 0.28%
• MgO : 00.12%
For a further explanation of Plaster of Paris in aquariums and Ponds, please read this article:
• THE DIFFERENCE BETWEEN PLASTER OF PARIS AND PRODUCTS SUCH AS WONDER SHELLS
Electrolytes
This section is somewhat redundant, as the positive mineral ions mentioned in the GH, Calcium and other sections essentially are the main electrolytes we will discuss in more depth here.
Calcium and Magnesium along potassium and Sodium make up essential “Reducing Electrolytes” while Chloride and Phosphates are generally oxidizing electrolytes (PO4; is an oxidizing agent but a very poor one). Generally, Calcium and Magnesium are associated with hard water while Sodium, Potassium, and Phosphates are associated with soft water. Potassium is also found in AAP/SeaChem Replenish.
What are Electrolytes? Electrolytes are molecular substances containing free ions which behave as an electrically conductive medium. In fish (or other living things) the primary ions of electrolytes are sodium (Na+), potassium (K+), calcium (Ca++), magnesium (Mg++), chloride (Cl-), phosphate (PO4---), and hydrogen carbonate (HCO3-).
Fish and other aquatic life forms such as shrimp require a subtle and complex electrolyte balance between the intracellular (inside the cell) and extra cellular (outside the cell such as plasma membranes) environment. In particular, the maintenance of precise osmotic gradients of electrolytes is important.
These gradients affect and regulate the hydration of the fish, blood pH, and disease resistance and are important for proper nerve and muscle function.
Sudden changes in these electrolytes (as with pH) can be stressful/detrimental as well, this includes rapid positive changes. For this reason, any aquarium that is depleted of positive mineral ions in particular, should have these introduced slowly. This is especially the case with many Labyrinth fish (such as Bettas), and Crystal Red Shrimp even though both benefit greatly from these mineral cations.
A fish or invertebrate stressed by the introduction of necessary positive ions is not evidence that these cations should avoided, rather proof of an unhealthy mineral cation depleted environment (as per good scientific reasoning). To avoid this stress, I would suggest a 1/4 to 1/2 dose of AAP/SeaChem Replenish/Trace, Wonder Shells, etc. if kept in a previously ion depleted environment.
Salt is another type of electrolyte commonly used in a dose of one tablespoon per 5 gallons (20 liters) in freshwater aquariums. This is one way to add some sodium electrolytes to a freshwater aquarium, but not the only.
Please Read this article prior to using salt in a freshwater aquarium:
• Salt use in Freshwater Aquariums
Do NOT make the mistake with a freshwater aquarium of using home softener water or products that replace calcium/magnesium ions with sodium ions (example API Water Softening Pillows), as this is creates water totally out of balance with essential electrolytes. The salt used produces water vastly too high in sodium and this high sodium water will precipitate out ALL ESSENTIAL positive calcium ions.
Adding these minerals back in via Wonder Shells, Trace/Replenish, Oolitic sands, etc. will not correct this long term at all as these minerals will continue to be precipitated out by the softened water.
Also do not make the mistake of comparing water softened via a home/office water softener to naturally soft water such as found in the Amazon River, the chemistry is not at all the same.
If Home water softener water is used, it must slowly be changed out.
See:
• Home/Office Water Softeners for Fish
Products such as SeaChem Replenish adds essential Electrolytes, more so in the “soft” electrolytes and in more trace amounts. Wonder Shells also adds electrolytes (especially the essential Redox Reducing “Hard” electrolytes). The advantage of mineral blocks such as the slow dissolving Wonder Shells is these electrolytes are added over longer sustained periods (important for mineral cation depletion) and even though more “hard” are still quite useful if only in half doses for more soft water fish such as Bettas, especially when combined with natural “long term softeners such as Indian Almond Leaves, Peat, Pillow/Frog Moss, Driftwood, etc.)
Salt will also aid in disease prevention as it will help with the generation of the slime coat (or Mucous) on fish as salt acts on the osmotic gradient. But care needs to be given with salt sensitive fish such as catfish not to exceed this amount. Another aspect of salt is that although it aids in the prevention and even treatment of Freshwater Ich (Ichthyophthirius multifilis), it is often poor treatment for a full-blown infection.
Reference:
• Treatment, Prevention of Aquarium Ich
The importance of electrolytes/trace elements in Marine aquariums is magnified since most marine (saltwater) drink the water around them. Depletion of these trace elements through biological processes needs to be compensated for by water changes using a quality marine mix AND by testing your alkalinity (KH)/Calcium/Magnesium and adding buffers such as Sea Chem Buffer accordingly that not only add necessary carbonates for buffering but also add necessary electrolytes such as Chloride, Calcium, and Magnesium to name a few.
It is noteworthy that the presence of many mineral ions (cations) can lower the toxicity of nitride anions that can be present in aquarium. This is one more reason for their importance.
Finally, while out of the scope of this article, the process of TRUE level one UV Sterilization can remove dangerous oxidizers to the water column. While a good UV Sterilizer does not add any positive electrolytes to the water, they do remove some negative anions, which in turn allows for less depletion of essential mineral ions from normal biological processes.
Reference:
• TRUE Level 1 UV Sterilization
Product Resources:
• Wonder Shells
• AAP/SeaChem Replenish
•UV Sterilizers; All Capable of Level 1 UVC Sterilization
For MUCH more on the effects of electrolytes and their function in freshwater and saltwater:
• How do Fish Drink; Proper Osmotic Function
Calcium and Magnesium along potassium and Sodium make up essential “Reducing Electrolytes” while Chloride and Phosphates are generally oxidizing electrolytes (PO4; is an oxidizing agent but a very poor one). Generally, Calcium and Magnesium are associated with hard water while Sodium, Potassium, and Phosphates are associated with soft water. Potassium is also found in AAP/SeaChem Replenish.
What are Electrolytes? Electrolytes are molecular substances containing free ions which behave as an electrically conductive medium. In fish (or other living things) the primary ions of electrolytes are sodium (Na+), potassium (K+), calcium (Ca++), magnesium (Mg++), chloride (Cl-), phosphate (PO4---), and hydrogen carbonate (HCO3-).
Fish and other aquatic life forms such as shrimp require a subtle and complex electrolyte balance between the intracellular (inside the cell) and extra cellular (outside the cell such as plasma membranes) environment. In particular, the maintenance of precise osmotic gradients of electrolytes is important.
These gradients affect and regulate the hydration of the fish, blood pH, and disease resistance and are important for proper nerve and muscle function.
Sudden changes in these electrolytes (as with pH) can be stressful/detrimental as well, this includes rapid positive changes. For this reason, any aquarium that is depleted of positive mineral ions in particular, should have these introduced slowly. This is especially the case with many Labyrinth fish (such as Bettas), and Crystal Red Shrimp even though both benefit greatly from these mineral cations.
A fish or invertebrate stressed by the introduction of necessary positive ions is not evidence that these cations should avoided, rather proof of an unhealthy mineral cation depleted environment (as per good scientific reasoning). To avoid this stress, I would suggest a 1/4 to 1/2 dose of AAP/SeaChem Replenish/Trace, Wonder Shells, etc. if kept in a previously ion depleted environment.
Salt is another type of electrolyte commonly used in a dose of one tablespoon per 5 gallons (20 liters) in freshwater aquariums. This is one way to add some sodium electrolytes to a freshwater aquarium, but not the only.
Please Read this article prior to using salt in a freshwater aquarium:
• Salt use in Freshwater Aquariums
Do NOT make the mistake with a freshwater aquarium of using home softener water or products that replace calcium/magnesium ions with sodium ions (example API Water Softening Pillows), as this is creates water totally out of balance with essential electrolytes. The salt used produces water vastly too high in sodium and this high sodium water will precipitate out ALL ESSENTIAL positive calcium ions.
Adding these minerals back in via Wonder Shells, Trace/Replenish, Oolitic sands, etc. will not correct this long term at all as these minerals will continue to be precipitated out by the softened water.
Also do not make the mistake of comparing water softened via a home/office water softener to naturally soft water such as found in the Amazon River, the chemistry is not at all the same.
If Home water softener water is used, it must slowly be changed out.
See:
• Home/Office Water Softeners for Fish
Products such as SeaChem Replenish adds essential Electrolytes, more so in the “soft” electrolytes and in more trace amounts. Wonder Shells also adds electrolytes (especially the essential Redox Reducing “Hard” electrolytes). The advantage of mineral blocks such as the slow dissolving Wonder Shells is these electrolytes are added over longer sustained periods (important for mineral cation depletion) and even though more “hard” are still quite useful if only in half doses for more soft water fish such as Bettas, especially when combined with natural “long term softeners such as Indian Almond Leaves, Peat, Pillow/Frog Moss, Driftwood, etc.)
Salt will also aid in disease prevention as it will help with the generation of the slime coat (or Mucous) on fish as salt acts on the osmotic gradient. But care needs to be given with salt sensitive fish such as catfish not to exceed this amount. Another aspect of salt is that although it aids in the prevention and even treatment of Freshwater Ich (Ichthyophthirius multifilis), it is often poor treatment for a full-blown infection.
Reference:
• Treatment, Prevention of Aquarium Ich
The importance of electrolytes/trace elements in Marine aquariums is magnified since most marine (saltwater) drink the water around them. Depletion of these trace elements through biological processes needs to be compensated for by water changes using a quality marine mix AND by testing your alkalinity (KH)/Calcium/Magnesium and adding buffers such as Sea Chem Buffer accordingly that not only add necessary carbonates for buffering but also add necessary electrolytes such as Chloride, Calcium, and Magnesium to name a few.
It is noteworthy that the presence of many mineral ions (cations) can lower the toxicity of nitride anions that can be present in aquarium. This is one more reason for their importance.
Finally, while out of the scope of this article, the process of TRUE level one UV Sterilization can remove dangerous oxidizers to the water column. While a good UV Sterilizer does not add any positive electrolytes to the water, they do remove some negative anions, which in turn allows for less depletion of essential mineral ions from normal biological processes.
Reference:
• TRUE Level 1 UV Sterilization
Product Resources:
• Wonder Shells
• AAP/SeaChem Replenish
•UV Sterilizers; All Capable of Level 1 UVC Sterilization
For MUCH more on the effects of electrolytes and their function in freshwater and saltwater:
• How do Fish Drink; Proper Osmotic Function
pH
What is pH? I will give a brief explanation since there is a lot of good more in-depth explanations elsewhere on the Internet. pH is most simply stated as a measurement of hydrogen ions (in other words their charge) in a logarithm to base 10. This can remain high until enough acids counter this charge or a resin is used to counter this charge. RO water does not alter this charge much, only DI water (hence the term de-ionized water).
More from Reef Aquarium Chemistry Maintenance: "A little about pH since alkalinity is what generally stabilizes pH in our aquariums. pH = a measurement of H+ and the more H+ the lower the pH and less Alkalinity in short. Molar value wise, it takes twice as much as Bicarbonate as Carbonate to raise the Alkalinity up 1 Equilibrium unit. Volume wise it is 0.6 tsp of Bicarbonate vs. 0.4 tsp of Carbonate to raise the Alkalinity 1 milliequivalent (mEq) / or 2.8 dKH in 10 gals."
PH is unfortunately often an area of misinformation with too much importance is give to “exact” pH numbers. As well unstable pH numbers often indicate other issues such as high amounts of decomposing organic mulm/high DOC.
Often aquarists will “chase” pH trying to achieve the “perfect” pH not realizing that pH stability is generally far more important than the actual pH number. Fish can adapt to different pH, however most fish are NOT tolerant to wide swings in pH. There is a simple explanation to this that many are not aware of, that is the pH scale is logarithmic, meaning a change of pH from say 7.0 to 6.0 is a tenfold increase in acidity, while a change from 7.0 to 5.0 is 100 times change in acidity (it also goes the other direction as to alkalinity).
This is also why care must be taken during water changes not to suddenly alter the pH by the addition of dramatically different pH water back into the aquarium. This can be a problem with high bio load aquariums due to large amounts of decomposing mulm that slowly reduces pH between water changes.
The use of products such as the Eheim Sludge Remover are useful for tanks with high bio loads so as to remove waste in between water changes and lower the risk of sudden pH shifts during subsequent water changes.
Product Resource:
• Eheim Sludge Remover, Battery Vacuum
Potential pH shock is also an important reason to not only float your new fish for temperature adjustment, but to also slowly introduce aquarium water to your new fish’ shipping bag prior to tank introduction.
Diagram above from:
• Elmhurst Education; pH
Another aspect of pH to be aware of is temperature. A higher temperature will lower pH readings at higher values (more alkaline, so it is important to compare pH readings at the same temperature, otherwise readings can be inaccurate. HOWEVER: "As the pH falls as temperature increases, this does not mean that water becomes more acidic at higher temperatures. A solution is acidic if there is an excess of hydrogen ions over hydroxide ions. In the case of pure water, there are always the same concentration of hydrogen ions and hydroxide ions and hence, the water is still neutral (pH = pOH) - even if its pH changes."
Above Referenced/Quoted in part from:
• Temperature Dependent of the pH of pure Water
KH as noted earlier is a stabilizer of pH (towards the alkaline side) whereas peat, Aquarium Driftwood, Pillow Moss, and dried almond leaves (such as Bio Lif) will aid in lower stabilization. As well, for really troublesome high pH aquariums, cutting pH/KH with blended RO water may be necessary (see later in this article).
Please note that Natural Buffers such as Peat, Driftwood, Pillow Moss, etc. generally lower pH via a slow release of acids such as Tannins. If your carbonates are high (KH) your pH will remain high until a slow balance is reached (please read more about this in the Amazon River Water section). To reach an acid/carbonate balance more quickly, products such as SeaChem’s Acid Buffer can be used.
If using Indian Almond Leaves, Pillow Moss, or Peat, I suggest at least temporarily suspending the use of carbon, you can still use it, just not after initial use of these natural acid buffers, as Carbon can remove Tannins. If the water gets too brown from these products, then slowly re-introduce the carbon back in.
Product Resources:
• Real Driftwood for Aquarium
• Pillow Moss for Natural Aquarium Acid Buffering
• Dried Indian Almond Leaf
More in Depth pH Information: The main point I would like to make as to pH is that often too much concern is given to a perfect pH when in reality a stable pH is more important, which I can speak to in the 1000s of aquariums I have maintained at different pH and other parameters. As an example, I have seen Discus (a fish which comes from waters often under 6.5) breed in aquariums with a pH of 7.5!!!
What is stressful is a pH that is not stable therefore a good KH and/or acid buffer is important. I have found electrolytes such as calcium are FAR MORE IMPORTANT than a so-called perfect pH (these will also affect a healthy Redox Potential).
The discus under my care were much healthier with a KH of around 50 ppm and often higher, a GH around 100-200 ppm and also often even higher, and a BALANCED Redox Potential that allows for both oxidation AND REDUCTION than with a pH of 6.5- 7.0 (please note that these numbers just given are for discus, for many fish such as livebearers I kept a much higher KH and GH). Ditto goes for Bettas, which are fish I probably read more incorrect information about than any as to what is important to these fish as per water chemistry (GH, KH, pH, & Redox in particular).
An exception to not chasing pH or a pH being lower in general in freshwater aquariums (not saltwater) is with some fish, a slow change in pH can be a signal of change of season and then be a breeding stimulant. By slowly decreasing pH over a few days in an aquarium this can often stimulate fish to breed. So, this is one aspect of aquarium chemistry where I would suggest a lower pH, but even then, only cyclically, in other words lowering an aquarium from 7.5 to 6.5 or even 6.0 for species such as been reported with American Flag fish. This also does NOT mean lowering GH and driving out mineral Cations is needed, only a lowering of pH as in nature as a signal of season change.
Another point to pH is tap water or well water pH when drawn will often gas out (sometimes referred to as “gassing out”). This is trapped CO2 gas in the tap water that will slowly gas out of the water if allowed to sit (also Hydrogen Sulfite can be present).
What I mean is you will get a sample of tap/well water then immediately test it and get a result such as 6.5 that can rise to 7.0 or higher over the next hour as CO2 gasses out, assuming there are carbonates (KH) in the water (even more common in well water from my experience). This is noteworthy in testing your tap water as you will NOT get accurate tap water pH readings if you test your water immediately after drawing it from the tap, it is best to wait at least one hour. This gassing out does NOT affect GH or KH as these are minerals that remain in the water.
The addition of mineral blocks such as Wonder Shells would not cause a problem as these would not add more carbonates to water already containing carbonates at level of 150 ppm KH for example. Again, stability is the key point and adding phosphate containing pH lowering products (such as “pH Down”) will tend to cause roller coaster pH swings in an established tank.
Also be aware that water conditioners are reducers and can have an affect on pH (generally higher), so do not test pH immediately after using ANY water conditioner.
More from Reef Aquarium Chemistry Maintenance: "A little about pH since alkalinity is what generally stabilizes pH in our aquariums. pH = a measurement of H+ and the more H+ the lower the pH and less Alkalinity in short. Molar value wise, it takes twice as much as Bicarbonate as Carbonate to raise the Alkalinity up 1 Equilibrium unit. Volume wise it is 0.6 tsp of Bicarbonate vs. 0.4 tsp of Carbonate to raise the Alkalinity 1 milliequivalent (mEq) / or 2.8 dKH in 10 gals."
PH is unfortunately often an area of misinformation with too much importance is give to “exact” pH numbers. As well unstable pH numbers often indicate other issues such as high amounts of decomposing organic mulm/high DOC.
Often aquarists will “chase” pH trying to achieve the “perfect” pH not realizing that pH stability is generally far more important than the actual pH number. Fish can adapt to different pH, however most fish are NOT tolerant to wide swings in pH. There is a simple explanation to this that many are not aware of, that is the pH scale is logarithmic, meaning a change of pH from say 7.0 to 6.0 is a tenfold increase in acidity, while a change from 7.0 to 5.0 is 100 times change in acidity (it also goes the other direction as to alkalinity).
This is also why care must be taken during water changes not to suddenly alter the pH by the addition of dramatically different pH water back into the aquarium. This can be a problem with high bio load aquariums due to large amounts of decomposing mulm that slowly reduces pH between water changes.
The use of products such as the Eheim Sludge Remover are useful for tanks with high bio loads so as to remove waste in between water changes and lower the risk of sudden pH shifts during subsequent water changes.
Product Resource:
• Eheim Sludge Remover, Battery Vacuum
Potential pH shock is also an important reason to not only float your new fish for temperature adjustment, but to also slowly introduce aquarium water to your new fish’ shipping bag prior to tank introduction.
Diagram above from:
• Elmhurst Education; pH
Another aspect of pH to be aware of is temperature. A higher temperature will lower pH readings at higher values (more alkaline, so it is important to compare pH readings at the same temperature, otherwise readings can be inaccurate. HOWEVER: "As the pH falls as temperature increases, this does not mean that water becomes more acidic at higher temperatures. A solution is acidic if there is an excess of hydrogen ions over hydroxide ions. In the case of pure water, there are always the same concentration of hydrogen ions and hydroxide ions and hence, the water is still neutral (pH = pOH) - even if its pH changes."
Above Referenced/Quoted in part from:
• Temperature Dependent of the pH of pure Water
KH as noted earlier is a stabilizer of pH (towards the alkaline side) whereas peat, Aquarium Driftwood, Pillow Moss, and dried almond leaves (such as Bio Lif) will aid in lower stabilization. As well, for really troublesome high pH aquariums, cutting pH/KH with blended RO water may be necessary (see later in this article).
Please note that Natural Buffers such as Peat, Driftwood, Pillow Moss, etc. generally lower pH via a slow release of acids such as Tannins. If your carbonates are high (KH) your pH will remain high until a slow balance is reached (please read more about this in the Amazon River Water section). To reach an acid/carbonate balance more quickly, products such as SeaChem’s Acid Buffer can be used.
If using Indian Almond Leaves, Pillow Moss, or Peat, I suggest at least temporarily suspending the use of carbon, you can still use it, just not after initial use of these natural acid buffers, as Carbon can remove Tannins. If the water gets too brown from these products, then slowly re-introduce the carbon back in.
Product Resources:
• Real Driftwood for Aquarium
• Pillow Moss for Natural Aquarium Acid Buffering
• Dried Indian Almond Leaf
More in Depth pH Information: The main point I would like to make as to pH is that often too much concern is given to a perfect pH when in reality a stable pH is more important, which I can speak to in the 1000s of aquariums I have maintained at different pH and other parameters. As an example, I have seen Discus (a fish which comes from waters often under 6.5) breed in aquariums with a pH of 7.5!!!
What is stressful is a pH that is not stable therefore a good KH and/or acid buffer is important. I have found electrolytes such as calcium are FAR MORE IMPORTANT than a so-called perfect pH (these will also affect a healthy Redox Potential).
The discus under my care were much healthier with a KH of around 50 ppm and often higher, a GH around 100-200 ppm and also often even higher, and a BALANCED Redox Potential that allows for both oxidation AND REDUCTION than with a pH of 6.5- 7.0 (please note that these numbers just given are for discus, for many fish such as livebearers I kept a much higher KH and GH). Ditto goes for Bettas, which are fish I probably read more incorrect information about than any as to what is important to these fish as per water chemistry (GH, KH, pH, & Redox in particular).
An exception to not chasing pH or a pH being lower in general in freshwater aquariums (not saltwater) is with some fish, a slow change in pH can be a signal of change of season and then be a breeding stimulant. By slowly decreasing pH over a few days in an aquarium this can often stimulate fish to breed. So, this is one aspect of aquarium chemistry where I would suggest a lower pH, but even then, only cyclically, in other words lowering an aquarium from 7.5 to 6.5 or even 6.0 for species such as been reported with American Flag fish. This also does NOT mean lowering GH and driving out mineral Cations is needed, only a lowering of pH as in nature as a signal of season change.
Another point to pH is tap water or well water pH when drawn will often gas out (sometimes referred to as “gassing out”). This is trapped CO2 gas in the tap water that will slowly gas out of the water if allowed to sit (also Hydrogen Sulfite can be present).
What I mean is you will get a sample of tap/well water then immediately test it and get a result such as 6.5 that can rise to 7.0 or higher over the next hour as CO2 gasses out, assuming there are carbonates (KH) in the water (even more common in well water from my experience). This is noteworthy in testing your tap water as you will NOT get accurate tap water pH readings if you test your water immediately after drawing it from the tap, it is best to wait at least one hour. This gassing out does NOT affect GH or KH as these are minerals that remain in the water.
The addition of mineral blocks such as Wonder Shells would not cause a problem as these would not add more carbonates to water already containing carbonates at level of 150 ppm KH for example. Again, stability is the key point and adding phosphate containing pH lowering products (such as “pH Down”) will tend to cause roller coaster pH swings in an established tank.
Also be aware that water conditioners are reducers and can have an affect on pH (generally higher), so do not test pH immediately after using ANY water conditioner.
Better is a stable GH and KH with the addition of Indian Almond Leaf Extract, Peat, Driftwood, or Pillow/Frog Moss to aid in the maintenance of a lower pH if desired. The use of an Acid Buffer along with carbonate (KH) buffers during initial set-up or water changes can provide more immediate results for low pH stability. Assuming a constant supply of carbonates (KH), the use of the above mentioned slow and “fast” acid buffers can also provide essential CO2 for planted aquariums (via a chemical reaction of the alkaline buffer and acid buffer).
Another common pH mistake, especially with fish kept in bowls such as Bettas is the use of RO water combined with too frequent of water changes along with little or no bio filtration and little or no mineral replenishment. It is noteworthy that RO water unlike DI water (which means DE-Ionized), may remove most all minerals and carbonates, resulting in a low GH & KH. HOWEVER, it DOES NOT alter pH much since many ions are still left after reverse osmosis unlike with DI water.
The result is a potentially still high pH that never stabilizes as it would normally in a healthy aquarium where by the natural nitrification process would lower pH when little KH is available to buffer (as it would be when RO water is used in full or in part). However the use of an Acid Buffer should immediately lower the pH in RO water sine little or no buffers would be present to counter act this process.
Product Resource (Recommended place to purchase to support this free information):
• Acid Buffer from AAP
Finally, another point that should be made as per pH that every aquarium keeper should be aware of is at a pH of 6.0 the process of nitrification changes (conversion by nitrifying bacteria of ammonia and nitrites). This results in total ammonia being converted to ammonium, which is nontoxic, but can easily suddenly change back to toxic NH3 ammonia. While on the other side of the equation most non-toxic ammonium (NH4) converts to toxic ammonia (NH3) at a pH of 8.0.
Simply put, a bouncing pH can result in often unnoticed ammonia spikes!!! The use of products such as SeaChem Stability which uses facultative bacteria that can still "eat" wastes at lower pH of 6.5 or less where nitrification starts to slow substantially. SeaChem Prime would be a good product to bind ammonia (NH3).
See this reference for more:
• Aquarium Nitrogen Cycle
Product Resources:
• SeaChem Stability; Aquarium Nitrogen Cycle Aid
• SeaChem Prime; Converts ammonia into a bio-available nontoxic form
Another common pH mistake, especially with fish kept in bowls such as Bettas is the use of RO water combined with too frequent of water changes along with little or no bio filtration and little or no mineral replenishment. It is noteworthy that RO water unlike DI water (which means DE-Ionized), may remove most all minerals and carbonates, resulting in a low GH & KH. HOWEVER, it DOES NOT alter pH much since many ions are still left after reverse osmosis unlike with DI water.
The result is a potentially still high pH that never stabilizes as it would normally in a healthy aquarium where by the natural nitrification process would lower pH when little KH is available to buffer (as it would be when RO water is used in full or in part). However the use of an Acid Buffer should immediately lower the pH in RO water sine little or no buffers would be present to counter act this process.
Product Resource (Recommended place to purchase to support this free information):
• Acid Buffer from AAP
Finally, another point that should be made as per pH that every aquarium keeper should be aware of is at a pH of 6.0 the process of nitrification changes (conversion by nitrifying bacteria of ammonia and nitrites). This results in total ammonia being converted to ammonium, which is nontoxic, but can easily suddenly change back to toxic NH3 ammonia. While on the other side of the equation most non-toxic ammonium (NH4) converts to toxic ammonia (NH3) at a pH of 8.0.
Simply put, a bouncing pH can result in often unnoticed ammonia spikes!!! The use of products such as SeaChem Stability which uses facultative bacteria that can still "eat" wastes at lower pH of 6.5 or less where nitrification starts to slow substantially. SeaChem Prime would be a good product to bind ammonia (NH3).
See this reference for more:
• Aquarium Nitrogen Cycle
Product Resources:
• SeaChem Stability; Aquarium Nitrogen Cycle Aid
• SeaChem Prime; Converts ammonia into a bio-available nontoxic form
Correcting Unstable Low KH/pH
Too LOW pH or KH problems Corrections for Unstable KH; Raising pH: First start by testing your tap or well water (as stated earlier this can gas out so wait for 1 hour before testing). If your tap/well water is low in pH with little buffering KH, you will have problems maintaining stable or higher pH if that is desired. Areas that draw their water from rivers, especially that flow in boggy areas or are of volcanic origin may have very low pH/KH. If tap/well water is the problem or part of the problem, you will need to add a buffer.
A buffer such as AAP/SeaChem Malawi Buffer is a blend of carbonate salts designed to increase carbonate hardness, buffer capacity, & pH. For planted aquariums I prefer the AAP/SeaChem Alkaline Buffer, although this product is not as stable as marine or rift lakes buffer as this buffer is more basic like Baking Soda so regular checks of KH are more important when this buffer is employed.
However, as noted in the KH section, the use of phosphate based products such as neutral regulator SHOULD BE AVOIDED!
While Wonder shells will help with Calcium, GH, & essential positive mineral ions; these are NOT a solution to a unstable pH/KH. At best these only aid in KH maintenance, however they are not as useful for water that is already very low in KH/pH and needs to be brought up.
As noted in the KH section, when a buffer is added to an aquarium with a KH below 50-100, while at the same time there are little acid buffers (either natural or chemical), the pH may bounce. DO NOT Chase the pH, simply add some form of acid buffer (many are described later too) and/or accept your new pH.
A tank with stable pH of 7.3 and KH of 50-80 for a Betta is better than one with a KH of under 50 and pH of 6.8!!!
If the tap/well water is OK or adjusted to preferred KH/pH yet continues to drop rapidly, I would look at tank conditions. Here are a few possible problems that cause pH/KH drops to look into: (Please note that these problems are often interrelated):
• Too high of DOC (dissolved organic compounds) caused by organic debris/mulm, high fish loads, etc. This can be spotted by a KH of (for example) that starts out at 80 ppm after cleaning, addition of buffer, and/or correct out of the tap, however KH drops rapidly after the initial measurements. As well, another symptom is that often ammonia, nitrites, and eventually nitrates will spike or be unstable.
Increasing the frequency of filter media rinsings (in cool de-chlorinated water though, so as to preserve nitrifying bacteria). This may mean rinsing as often as twice a week and this includes filter media inside bio bags (Whisper), carbon inserts (Aqua Clear, Internal Wet/Dry), etc. Also increasing the amount of buffers added to maintain a stable KH will help (assuming ammonia spikes do not indicate a more serious issue).
Lowering fish levels or other aquarium animal inhabitant populations will most certainly help. As well, watch for snail population "explosions", especially small snails living in filters, under gravel, etc. as these can be a hidden cause of acid producing nitrification and decomposition.
• Mulm buildup under rocks/décor and in canister filters/Wet-Dry filters. Canister filters in particular if let go without a cleaning more than 6 weeks can build up a lot of decomposing mulm that will add acids to your water. The use of SeaChem Purigen can absorb organic compounds prior nitrification and/or decomposition. As well SeaChem Matrix added to filters can balance the nitrification process with de-nitrification.
Product Resources:
• SeaChem Purigen; Removes Nitrogenous Organic Waste
• SeaChem Matrix; High porosity Bio Media
• Too much Driftwood/Mango Wood; Some driftwood in particular can be full of tannins that will lower pH/KH. Also decomposing (rotting) driftwood will lower pH and KH more than normal cured driftwood, often a symptom of this besides pH drops includes ammonia, nitrite and eventually nitrate spikes.
• Too deep a sand bed, this again can add to decomposition that leads to acid build up (sand tends to worse than gravel here, however gravel can cause this too when too deep. Even an otherwise normally good #3 grade of gravel that is regularly vacuumed may still harbor organic mulm, this is particularly true of many epoxy coated colored gravels as this coating often starts to loosen and allow organic mulm to be trapped there resulting in rapid KH depletion! The solution is to often remove ½ the gravel at a time (so as to cause as little interruption of the nitrogen cycle) and either change (usually in the case of colored gravel) or thoroughly wash it.
Further Reference:
• Aquarium Gravel, Substrate
• Infrequent or poorly conducted water changes/cleanings.
Please see this article for more:
• Aquarium Cleaning; Methods & Reasons
After correcting these problems, you still may need to add a buffer or aragonite bag to your aquarium.
A buffer such as AAP/SeaChem Malawi Buffer is a blend of carbonate salts designed to increase carbonate hardness, buffer capacity, & pH. For planted aquariums I prefer the AAP/SeaChem Alkaline Buffer, although this product is not as stable as marine or rift lakes buffer as this buffer is more basic like Baking Soda so regular checks of KH are more important when this buffer is employed.
However, as noted in the KH section, the use of phosphate based products such as neutral regulator SHOULD BE AVOIDED!
While Wonder shells will help with Calcium, GH, & essential positive mineral ions; these are NOT a solution to a unstable pH/KH. At best these only aid in KH maintenance, however they are not as useful for water that is already very low in KH/pH and needs to be brought up.
As noted in the KH section, when a buffer is added to an aquarium with a KH below 50-100, while at the same time there are little acid buffers (either natural or chemical), the pH may bounce. DO NOT Chase the pH, simply add some form of acid buffer (many are described later too) and/or accept your new pH.
A tank with stable pH of 7.3 and KH of 50-80 for a Betta is better than one with a KH of under 50 and pH of 6.8!!!
If the tap/well water is OK or adjusted to preferred KH/pH yet continues to drop rapidly, I would look at tank conditions. Here are a few possible problems that cause pH/KH drops to look into: (Please note that these problems are often interrelated):
• Too high of DOC (dissolved organic compounds) caused by organic debris/mulm, high fish loads, etc. This can be spotted by a KH of (for example) that starts out at 80 ppm after cleaning, addition of buffer, and/or correct out of the tap, however KH drops rapidly after the initial measurements. As well, another symptom is that often ammonia, nitrites, and eventually nitrates will spike or be unstable.
Increasing the frequency of filter media rinsings (in cool de-chlorinated water though, so as to preserve nitrifying bacteria). This may mean rinsing as often as twice a week and this includes filter media inside bio bags (Whisper), carbon inserts (Aqua Clear, Internal Wet/Dry), etc. Also increasing the amount of buffers added to maintain a stable KH will help (assuming ammonia spikes do not indicate a more serious issue).
Lowering fish levels or other aquarium animal inhabitant populations will most certainly help. As well, watch for snail population "explosions", especially small snails living in filters, under gravel, etc. as these can be a hidden cause of acid producing nitrification and decomposition.
• Mulm buildup under rocks/décor and in canister filters/Wet-Dry filters. Canister filters in particular if let go without a cleaning more than 6 weeks can build up a lot of decomposing mulm that will add acids to your water. The use of SeaChem Purigen can absorb organic compounds prior nitrification and/or decomposition. As well SeaChem Matrix added to filters can balance the nitrification process with de-nitrification.
Product Resources:
• SeaChem Purigen; Removes Nitrogenous Organic Waste
• SeaChem Matrix; High porosity Bio Media
• Too much Driftwood/Mango Wood; Some driftwood in particular can be full of tannins that will lower pH/KH. Also decomposing (rotting) driftwood will lower pH and KH more than normal cured driftwood, often a symptom of this besides pH drops includes ammonia, nitrite and eventually nitrate spikes.
• Too deep a sand bed, this again can add to decomposition that leads to acid build up (sand tends to worse than gravel here, however gravel can cause this too when too deep. Even an otherwise normally good #3 grade of gravel that is regularly vacuumed may still harbor organic mulm, this is particularly true of many epoxy coated colored gravels as this coating often starts to loosen and allow organic mulm to be trapped there resulting in rapid KH depletion! The solution is to often remove ½ the gravel at a time (so as to cause as little interruption of the nitrogen cycle) and either change (usually in the case of colored gravel) or thoroughly wash it.
Further Reference:
• Aquarium Gravel, Substrate
• Infrequent or poorly conducted water changes/cleanings.
Please see this article for more:
• Aquarium Cleaning; Methods & Reasons
After correcting these problems, you still may need to add a buffer or aragonite bag to your aquarium.
Amazon River, SE Asia, Too High pH
For Fish such as Discus, Bettas, German Rams, Apistos (Apistogrammas).
This section not only discusses method for lowering pH & KH but providing for a "softer" mineral water environment, assuming this is truly necessary based on what has been explained and documented to this point in the article, especially since much of the concern over GH in particular has not been scientifically proven, and in fact quite the opposite has been shown as per needs for mineral Cations and how GH truly affects fish.
From the GH Section of this article: More importantly as to respiration, the surface epithelia of gills and body surfaces are protected from direct interaction with the environment by mucous and intercellular junctions. Fish mucous has been postulated to have calcium binding properties. Mucous is a glycoprotein and could serve as a calcium chelating agent retarding ion loss from epithelial cells as a charged surface coat or barrier and thus is dependent on calcium for normal function. So please read this entire section with this in mind.
This section not only discusses method for lowering pH & KH but providing for a "softer" mineral water environment, assuming this is truly necessary based on what has been explained and documented to this point in the article, especially since much of the concern over GH in particular has not been scientifically proven, and in fact quite the opposite has been shown as per needs for mineral Cations and how GH truly affects fish.
From the GH Section of this article: More importantly as to respiration, the surface epithelia of gills and body surfaces are protected from direct interaction with the environment by mucous and intercellular junctions. Fish mucous has been postulated to have calcium binding properties. Mucous is a glycoprotein and could serve as a calcium chelating agent retarding ion loss from epithelial cells as a charged surface coat or barrier and thus is dependent on calcium for normal function. So please read this entire section with this in mind.
For areas with high KH or pH problems the use of RO or DI water blended with tap or well water is simple and effective method to solve this "problem" (sometimes combined with pH/KH buffers discussed later).
Do NOT use products such as pH down! Simply adding sodium bisulphate (NaHSO4) will lower the pH but will NOT keep a stable pH for which stability is much more important than the actual pH number for most fish, including the softer water fish we are discussing in this section.
As well, DO NOT use water from a home/business water softener as this "artificial" softwater will drive out ALL mineral cations, no matter how much you add back in. As noted in the "Electrolytes" section of this article, the chemistry of water softened by a water softener is not at all the same as soft water from the Amazon or most any other natural soft body of water. These waters are softened by tannins, not the use of sodium or potassium to drive out all other minerals via precipitation.
The difference in the simplest terms is water in SE Asia or the Amazon is softened naturally by tannins in peat, etc. These tannins basically out compete the mineral cations that are present in the water, but these mineral cations are still present as in the Amazon River.
Use of RO (Reverse Osmosis) and/or DI (De-ionized) Water (or very low GH Tap/Well Water): RO (Reverse Osmosis) or DI (De-ionized) water can be used to aid in pH, GH, & KH reduction. The process of blending RO with tap or well water is also called "Cutting Water". Other than advanced aquarium keepers, I would recommend using or starting with no more than 25% to start with the remaining 75% well or tap water. Higher amounts of RO/DI water can be used later as needed and as the aquarium keeper slowly finds the "sweet spot".
Example: If your tap water is say, 8.0 pH with a KH of 200 ppm, the addition of 25% RO/DI water (neutral 7.0 ph, 0 KH) should cut your KH by 25% to 150 KH, and your pH to 7.7. There are many other variables such as substrate, bio load, that makes this not as simple as I would wish this to be for non-advanced aquarium keepers; but for those seeking simple adjustments for community aquariums or basic Discus, Betta, etc. fish keeping, this is still a reasonably stress free simple solution that also does not require the purchase of any products other than a few bottles of RO or RO/DI water. You may have to experiment some such as using 50% RO water, but once you find a percentage, unless your tap water chemistry changes (which is more common that you may think), your results should then be consistent.
Prior to introducing the RO (or DI) water to "Cut" your tap water, I often recommend the addition of essential mineral ions such as by use these methods/products, to the amount of RO/DI water used (unless GH is also altready quite high even when "blended"):
• Soaking an AAP Wonder Shell in the RO water.
• API Electro-Right.
• Kent RO Right.
• SeaChem Replenish.
• Tropic Marin Pro Discus Mineral. (The latter two being the better products being the best in my experience.)
Often SeaChem Equilibrium is recommended in place of SeaChem Replenish for planted aquariums, however this is where I respectfully disagree with SeaChem based on my experience & research.
The reason is while it is true that sodium can build up and harm some plants, the purpose is not for ongoing use as I suggest, but a one time charge to add ALL essential mineral electrolytes to your aquarium, then maintain with AAP Wonder Shells (which have basically NO sodium).
Product Resources (Recommended place to purchase to support this free information):
• AAP Wonder Shells
• Professional Reverse Osmosis Systems for Aquarium
• SeaChem Replenish; Replaces Essential Minerals Removed by Reverse Osmosis
Sometimes Alkaline Buffers will need to be added to your RO/DI water, however often when blending with tap/well water to a percentage that hits the "sweet spot" you are looking for as per KH, this will not be necessary. However, if using closer to 100% RO/DI, alkaline buffers likely will be needed.
For advanced aquarists that are confident in their use of buffers, mineral and electrolyte replacements may use a higher percentage or even 100% RO/DI.
See:
• Use of RO or DI Water in Aquariums.
You can adjust this percentage up with subsequent water changes as you find your “sweet spot” in KH & pH but remember that pH stability is often much more important than the actual number. Also be careful in using bottled drinking water assuming it has proper minerals, it usually does not.
RO or DI System? As for what is is better to use, RO or DI, the short answer is if an optimum Reverse Osmosis Filter/Unit is used that utilizes quality TFC (thin film composite) membranes such as the premium Pentair medical grade membrane and activated catalytic carbon block filters, along with a build that properly utilizes the membrane without "flow-by", then the RO unit is more than adequate!
Unfortunately many persons read the "cut and past" hype in many forums, then fall for the cheap price of many Amazon/eBay combined RO/DI units that often cost less than a good quality 3 stage RO unit only.
However, when common sense logic is applied, if you are using an RO unit such as the AAP/TMC that applies the features already noted, you will get basically 0 nitrates, 0 phosphates, and 0 chlorine/chloramines and NOT get the concentrated ammonia or other molecules due to high rejection rates and inefficient membranes that then result in your DI resin chamber working overtime to removes these! The result is the need to replace these DI resins every 50 gallons of produced water or even less leading to operation cost that far exceed any real or imagined benefit of purchasing these lower cost combined RO/DI filters!!!
About all the DI (which means De-ionized) really does for an aquarium keeper over a well designed/quality RO unit is produce a neutral pH water. For marine reef tanks this is unnecessary based on my extensive use and comparison over the years. For a softwater Amazon biotope aquarium, since the KH buffers are stripped by the RO unit, the simple addition of an acid buffer or the natural nitrification bio processes will reduce this pH.
Product Resource (Recommended place to purchase to support this free information):
• Premium TMC/AAP Reverse Osmosis (RO) Filter/System
Minerals in Amazon, SE Asia, West Africa Water Aquariums: Calcium is also very important to Amazon basin fish as well as other fish too albeit in lesser quantities than say Mollies or Rift Lake Cichlids (despite some old school commentary to the contrary) and the lack of calcium minerals is not usually as evident in short term observations, however long-term health and disease resistance will suffer. Without a constant supply of trace amounts of calcium and other reducing mineral cations, this can result in diseases/ailments such as HITH disease in Oscars and other fish.
Reference:
• Aquarium Answers, Hole in the Head Disease
I should also note (despite common misunderstanding about water chemistry) that the addition of these mineral ions does not directly affect pH only GH.
Calcium should also be included, and many electrolyte replenishers (such as API Electrolyte & SeaChem Equilibrium) do NOT add adequate amounts of Calcium. Please note that I am not putting down such products, as I have used them and they are very good for replenishing some electrolytes including some chlorides, they are just not complete as per continual replenishment of positive calcium cations (necessary for Redox health). Original Wonder Shells can also be used with these products to provide better balance in this area, although when added to the aquarium I generally only use 1/4 the suggested size of Wonder Shell for Amazon River/SE Asia biotope water.
Product Resource:
• Original/Unique Wonder Shells
Do NOT use products such as pH down! Simply adding sodium bisulphate (NaHSO4) will lower the pH but will NOT keep a stable pH for which stability is much more important than the actual pH number for most fish, including the softer water fish we are discussing in this section.
As well, DO NOT use water from a home/business water softener as this "artificial" softwater will drive out ALL mineral cations, no matter how much you add back in. As noted in the "Electrolytes" section of this article, the chemistry of water softened by a water softener is not at all the same as soft water from the Amazon or most any other natural soft body of water. These waters are softened by tannins, not the use of sodium or potassium to drive out all other minerals via precipitation.
The difference in the simplest terms is water in SE Asia or the Amazon is softened naturally by tannins in peat, etc. These tannins basically out compete the mineral cations that are present in the water, but these mineral cations are still present as in the Amazon River.
Use of RO (Reverse Osmosis) and/or DI (De-ionized) Water (or very low GH Tap/Well Water): RO (Reverse Osmosis) or DI (De-ionized) water can be used to aid in pH, GH, & KH reduction. The process of blending RO with tap or well water is also called "Cutting Water". Other than advanced aquarium keepers, I would recommend using or starting with no more than 25% to start with the remaining 75% well or tap water. Higher amounts of RO/DI water can be used later as needed and as the aquarium keeper slowly finds the "sweet spot".
Example: If your tap water is say, 8.0 pH with a KH of 200 ppm, the addition of 25% RO/DI water (neutral 7.0 ph, 0 KH) should cut your KH by 25% to 150 KH, and your pH to 7.7. There are many other variables such as substrate, bio load, that makes this not as simple as I would wish this to be for non-advanced aquarium keepers; but for those seeking simple adjustments for community aquariums or basic Discus, Betta, etc. fish keeping, this is still a reasonably stress free simple solution that also does not require the purchase of any products other than a few bottles of RO or RO/DI water. You may have to experiment some such as using 50% RO water, but once you find a percentage, unless your tap water chemistry changes (which is more common that you may think), your results should then be consistent.
Prior to introducing the RO (or DI) water to "Cut" your tap water, I often recommend the addition of essential mineral ions such as by use these methods/products, to the amount of RO/DI water used (unless GH is also altready quite high even when "blended"):
• Soaking an AAP Wonder Shell in the RO water.
• API Electro-Right.
• Kent RO Right.
• SeaChem Replenish.
• Tropic Marin Pro Discus Mineral. (The latter two being the better products being the best in my experience.)
Often SeaChem Equilibrium is recommended in place of SeaChem Replenish for planted aquariums, however this is where I respectfully disagree with SeaChem based on my experience & research.
The reason is while it is true that sodium can build up and harm some plants, the purpose is not for ongoing use as I suggest, but a one time charge to add ALL essential mineral electrolytes to your aquarium, then maintain with AAP Wonder Shells (which have basically NO sodium).
Product Resources (Recommended place to purchase to support this free information):
• AAP Wonder Shells
• Professional Reverse Osmosis Systems for Aquarium
• SeaChem Replenish; Replaces Essential Minerals Removed by Reverse Osmosis
Sometimes Alkaline Buffers will need to be added to your RO/DI water, however often when blending with tap/well water to a percentage that hits the "sweet spot" you are looking for as per KH, this will not be necessary. However, if using closer to 100% RO/DI, alkaline buffers likely will be needed.
For advanced aquarists that are confident in their use of buffers, mineral and electrolyte replacements may use a higher percentage or even 100% RO/DI.
See:
• Use of RO or DI Water in Aquariums.
You can adjust this percentage up with subsequent water changes as you find your “sweet spot” in KH & pH but remember that pH stability is often much more important than the actual number. Also be careful in using bottled drinking water assuming it has proper minerals, it usually does not.
RO or DI System? As for what is is better to use, RO or DI, the short answer is if an optimum Reverse Osmosis Filter/Unit is used that utilizes quality TFC (thin film composite) membranes such as the premium Pentair medical grade membrane and activated catalytic carbon block filters, along with a build that properly utilizes the membrane without "flow-by", then the RO unit is more than adequate!
Unfortunately many persons read the "cut and past" hype in many forums, then fall for the cheap price of many Amazon/eBay combined RO/DI units that often cost less than a good quality 3 stage RO unit only.
However, when common sense logic is applied, if you are using an RO unit such as the AAP/TMC that applies the features already noted, you will get basically 0 nitrates, 0 phosphates, and 0 chlorine/chloramines and NOT get the concentrated ammonia or other molecules due to high rejection rates and inefficient membranes that then result in your DI resin chamber working overtime to removes these! The result is the need to replace these DI resins every 50 gallons of produced water or even less leading to operation cost that far exceed any real or imagined benefit of purchasing these lower cost combined RO/DI filters!!!
About all the DI (which means De-ionized) really does for an aquarium keeper over a well designed/quality RO unit is produce a neutral pH water. For marine reef tanks this is unnecessary based on my extensive use and comparison over the years. For a softwater Amazon biotope aquarium, since the KH buffers are stripped by the RO unit, the simple addition of an acid buffer or the natural nitrification bio processes will reduce this pH.
Product Resource (Recommended place to purchase to support this free information):
• Premium TMC/AAP Reverse Osmosis (RO) Filter/System
Minerals in Amazon, SE Asia, West Africa Water Aquariums: Calcium is also very important to Amazon basin fish as well as other fish too albeit in lesser quantities than say Mollies or Rift Lake Cichlids (despite some old school commentary to the contrary) and the lack of calcium minerals is not usually as evident in short term observations, however long-term health and disease resistance will suffer. Without a constant supply of trace amounts of calcium and other reducing mineral cations, this can result in diseases/ailments such as HITH disease in Oscars and other fish.
Reference:
• Aquarium Answers, Hole in the Head Disease
I should also note (despite common misunderstanding about water chemistry) that the addition of these mineral ions does not directly affect pH only GH.
Calcium should also be included, and many electrolyte replenishers (such as API Electrolyte & SeaChem Equilibrium) do NOT add adequate amounts of Calcium. Please note that I am not putting down such products, as I have used them and they are very good for replenishing some electrolytes including some chlorides, they are just not complete as per continual replenishment of positive calcium cations (necessary for Redox health). Original Wonder Shells can also be used with these products to provide better balance in this area, although when added to the aquarium I generally only use 1/4 the suggested size of Wonder Shell for Amazon River/SE Asia biotope water.
Product Resource:
• Original/Unique Wonder Shells
Use of RO/Di- Closed System
An aspect that GH & Calcium with Amazon River/ SE Asia Water that is missed by many (as per my email and other conversations) is that while the Amazon River may be a very low GH, this is an open environment where mineral cations are constantly replenished from the Andes Mountains. Similar is for the monsoonal flows in SE Asia (although not as regular).
However, an aquarium IS A CLOSED ENVIRONMENT and depletion of mineral cations can be very quick, especially when lower GH levels are maintained (see the earlier section about "Depletion of Positive Ions") and we already know the importances of GH.
This closed environment aspect is missed by many who promote methods such as Biotope or natural environment aquariums which simulate an environment that is closes to nature. While I am certainly not against a "natural" environment for Amazon River or similar type fish, just understand the importance of proper levels of mineral ions. No one should ignore the FACT that these fish still require mineral cations for proper health and Redox balance! Correct levels of mineral cations have been proven important in many places throughout my articles.
Unlike in an open system that have mountains and rivers replenishing these minerals, these mineral cations are often driven out with many methods of establishing a Biotope aquarium. The aquarium keeper is unlikely to know how important these proper trace minerals are for long term health of the fish.
Here is an example in saltwater world: For years many salt mixes and even sellers of actual saltwater for marine aquariums (including myself) advertised that their product was 99-100% like that of the ocean in chemical makeup. This is something you used to see, but you will no longer see this by better manufacturers.
This is not bragged about anymore as it has been well established in a closed saltwater aquarium system, that certain elements such as buffers and calcium must be in a higher supply as they are depleted much more quickly. I also found this out in experiments early in the 1980s where I used ocean water that was aged 30 days to prevent disease introduction thinking I would get better results when in reality the opposite turned out to be true. THIS ALSO APPLIES TO FRESHWATER!
So please, remember this when you set up your Biotope aquarium or any aquarium for that matter; make sure to supply at least some mineral cations, and do not use soft water or products that will precipitate out these mineral cations!!!
The bottom line is this closed environment aspect of an aquarium is missed by so many. For this reason, this is why I have achieved better results with partial or sometimes full RO water use along with Replenish & Alkaline Buffer initially followed with a regular 1/4 to 1/2 normal dose of Wonder Shell over products such as Equilibrium in an Amazon River biotope!
For those who still do not understand biochemistry as it applies to fish and all animals; and believe this is the only article that states the importance of calcium and other positive mineral ions for Discus, Bettas, German Rams, or even Shrimp. I suggest reading some of the research into Redox Balance (& Mamoon Kundi pHd work in the area of Redox Reduction) or read others such as Atison Phumchoosri or Marc Weiss (both well known for their work with soft water fish)!
References:
• The Importance of Aquarium Redox Balance
• Atison Phumchoosri
However, an aquarium IS A CLOSED ENVIRONMENT and depletion of mineral cations can be very quick, especially when lower GH levels are maintained (see the earlier section about "Depletion of Positive Ions") and we already know the importances of GH.
This closed environment aspect is missed by many who promote methods such as Biotope or natural environment aquariums which simulate an environment that is closes to nature. While I am certainly not against a "natural" environment for Amazon River or similar type fish, just understand the importance of proper levels of mineral ions. No one should ignore the FACT that these fish still require mineral cations for proper health and Redox balance! Correct levels of mineral cations have been proven important in many places throughout my articles.
Unlike in an open system that have mountains and rivers replenishing these minerals, these mineral cations are often driven out with many methods of establishing a Biotope aquarium. The aquarium keeper is unlikely to know how important these proper trace minerals are for long term health of the fish.
Here is an example in saltwater world: For years many salt mixes and even sellers of actual saltwater for marine aquariums (including myself) advertised that their product was 99-100% like that of the ocean in chemical makeup. This is something you used to see, but you will no longer see this by better manufacturers.
This is not bragged about anymore as it has been well established in a closed saltwater aquarium system, that certain elements such as buffers and calcium must be in a higher supply as they are depleted much more quickly. I also found this out in experiments early in the 1980s where I used ocean water that was aged 30 days to prevent disease introduction thinking I would get better results when in reality the opposite turned out to be true. THIS ALSO APPLIES TO FRESHWATER!
So please, remember this when you set up your Biotope aquarium or any aquarium for that matter; make sure to supply at least some mineral cations, and do not use soft water or products that will precipitate out these mineral cations!!!
The bottom line is this closed environment aspect of an aquarium is missed by so many. For this reason, this is why I have achieved better results with partial or sometimes full RO water use along with Replenish & Alkaline Buffer initially followed with a regular 1/4 to 1/2 normal dose of Wonder Shell over products such as Equilibrium in an Amazon River biotope!
For those who still do not understand biochemistry as it applies to fish and all animals; and believe this is the only article that states the importance of calcium and other positive mineral ions for Discus, Bettas, German Rams, or even Shrimp. I suggest reading some of the research into Redox Balance (& Mamoon Kundi pHd work in the area of Redox Reduction) or read others such as Atison Phumchoosri or Marc Weiss (both well known for their work with soft water fish)!
References:
• The Importance of Aquarium Redox Balance
• Atison Phumchoosri
Back to the process of producing an Amazon/ SE Asia like environment while maintaining essential mineral ions: Be patient with this process as you want slowly lower pH (and KH well), as earlier stated you can increase your percentage of RO or DI water each time you change water however you do not want stress fish by a large RO percentage right away.
Also keep in mind that Peat, Driftwood, Frog (Pillow) Moss (see picture to the left) will slowly work on pH and KH reduction, NOT overnight! Driftwood can be cut into increasingly smaller pieces for more water exposure to pH reducing tannins to increase effectiveness.
These products act as low pH buffers and will generally not bring down a pH immediately (nor do you want to either!), if your KH is especially high this will take time and subsequent water changes with blended RO water (which will “cut” the pH). However, you can boil peat in a pot or almond leaves to release all the acid faster.
Pillow moss is somewhat of an exception from my experiments as I have performed experiments with a 15-gallon aquarium utilizing two Frog Moss pieces; the Frog Moss reduced KH from 60 ppm to 10-20 ppm in one week.
In a related thought, if too many tannins are released, using less peat, peat, or driftwood may be advised (as noted earlier Driftwood can also be cut into pieces).
A true acid buffer such as SeaChem Acid Buffer can also be used for more immediate results, however it is often necessary to use such acid buffer products with either SeaChen Alkaline Buffer or even just baking soda so as to maintain a stable pH (and also provide CO2 for live plants if kept). I personally recommend the use of Acid Buffers only during set up and water changes, and recommend the use of Driftwood, Indian almond leaves, pillow moss, etc. for constant but slow acid buffers in the same way I recommend the use of Wonder Shells for a more constant supply of essential mineral ions (rather than products that provide a temporary supply of mineral ions such as Atison’s Spa).
Product Resources:
• Real Aquarium Driftwood Decorations
• AAP/SeaChem Bisulfate Salts Acid Buffer
• AAP/Pillow (Frog) Moss
As I noted earlier in the general pH section, although Peat, Pillow Moss, Driftwood, Bio-Lif or similar Almond Leaf products will lower pH, it is done via the slow production of acids such as tannins. If your tap or well water used is very high in carbonates or bicarbonates (which are KH buffers) you will see NO effect on your pH and you will need to lower/reduce these KH buffers (not totally eliminate) for these to be effective or allow for time as the tannins slowly dissipate the carbonates via interaction with the acids/tannins.
This is why I generally used blended RO/DI water (often with fast acid buffers such as SeaChems) as noted earlier for my water changes for my clients in the LA area where high carbonate tap-water was a way of life.
Water Changes: Once your pH and KH is where you want it, you want to adjust your RO or DI water as noted earlier prior to adding back to the aquarium. Ditto if you use tap/well water that is much higher in pH/KH than your aquarium water. This should be adjusted with the AAP/SeaChem Acid Buffer, Pillow Moss, etc. prior. to adding back to the aquarium after a water change. A 32-gallon RubberMaid trash can works for this (clean first with salt water before using).
Also keep in mind that Peat, Driftwood, Frog (Pillow) Moss (see picture to the left) will slowly work on pH and KH reduction, NOT overnight! Driftwood can be cut into increasingly smaller pieces for more water exposure to pH reducing tannins to increase effectiveness.
These products act as low pH buffers and will generally not bring down a pH immediately (nor do you want to either!), if your KH is especially high this will take time and subsequent water changes with blended RO water (which will “cut” the pH). However, you can boil peat in a pot or almond leaves to release all the acid faster.
Pillow moss is somewhat of an exception from my experiments as I have performed experiments with a 15-gallon aquarium utilizing two Frog Moss pieces; the Frog Moss reduced KH from 60 ppm to 10-20 ppm in one week.
In a related thought, if too many tannins are released, using less peat, peat, or driftwood may be advised (as noted earlier Driftwood can also be cut into pieces).
A true acid buffer such as SeaChem Acid Buffer can also be used for more immediate results, however it is often necessary to use such acid buffer products with either SeaChen Alkaline Buffer or even just baking soda so as to maintain a stable pH (and also provide CO2 for live plants if kept). I personally recommend the use of Acid Buffers only during set up and water changes, and recommend the use of Driftwood, Indian almond leaves, pillow moss, etc. for constant but slow acid buffers in the same way I recommend the use of Wonder Shells for a more constant supply of essential mineral ions (rather than products that provide a temporary supply of mineral ions such as Atison’s Spa).
Product Resources:
• Real Aquarium Driftwood Decorations
• AAP/SeaChem Bisulfate Salts Acid Buffer
• AAP/Pillow (Frog) Moss
As I noted earlier in the general pH section, although Peat, Pillow Moss, Driftwood, Bio-Lif or similar Almond Leaf products will lower pH, it is done via the slow production of acids such as tannins. If your tap or well water used is very high in carbonates or bicarbonates (which are KH buffers) you will see NO effect on your pH and you will need to lower/reduce these KH buffers (not totally eliminate) for these to be effective or allow for time as the tannins slowly dissipate the carbonates via interaction with the acids/tannins.
This is why I generally used blended RO/DI water (often with fast acid buffers such as SeaChems) as noted earlier for my water changes for my clients in the LA area where high carbonate tap-water was a way of life.
Water Changes: Once your pH and KH is where you want it, you want to adjust your RO or DI water as noted earlier prior to adding back to the aquarium. Ditto if you use tap/well water that is much higher in pH/KH than your aquarium water. This should be adjusted with the AAP/SeaChem Acid Buffer, Pillow Moss, etc. prior. to adding back to the aquarium after a water change. A 32-gallon RubberMaid trash can works for this (clean first with salt water before using).
Overview of Products for "Naturally" Softening Water.
Other products that are popular for softening water, lowering pH, and for creating an Amazon or Breeding environment include black water tonics and Atison’s SPA. I personally do not care for the old black water remedies as I find them to mostly work like the proverbial “chicken soup” placebo effect when put to actual tests and in fact I have seen harmful anaerobic bacteria added from these remedies.
Natural tannin products such as Pillow Moss along with a constant supply of calcium ions provided by a second product would be my first choice (based on the benefits of constant mineral ions/cations as pointed out earlier in the article).
Another choice is Atison’s Spa (which also contains calcium). However, this product sometimes fools users who understand the need for calcium, but miss the even more important fact that while Discus, Bettas, Rams and other Amazon/SE Asia Fish need small amounts of calcium, what is more important is the regular supply of positive calcium ion, albeit in small amounts. For this reason, I still strongly recommend a small but regular addition of mineral supplements whether liquid or in dissolving block form.
As to Atison’s SPA, this is growing in popularity among Betta enthusiasts due to mega internet site promotions. However, it uses almond leaves that are very refined which causes the loss of potency as compared to the superior pillow moss or the use of simply raw Almond Leaves. The Betta breeders in LA I know that previously used Bio Lif (now unfortunately discontinued) and now simply use raw Almond leaves or Pillow Moss along with constant mineral supplements and get good results, however these guys are not active in Betta or Discus groups/forums, so these methods are not nearly as well publicized as the use of Atison’s SPA.
The bottom line as per Atison's Betta Spa, is this is best used in small tanks/bowls where use of more advanced methods is often difficult.
Other choices include the before mentioned Pillow Moss, Peat, Driftwood pieces, etc. supplemented by a separate addition of minerals such as with Wonder Shells or a drip method of liquid calcium with products such as SeaChem Replenish in a volume of water which will add a constant supply of important mineral cations that are essential for fish or even water changes using high calcium water.
Finally, it is important as you also want to note that the Amazon River starts high in the Andes Mountains where it picks up a lot of minerals only to be buffer “down” by organics such as peat and bio-decay (as well as dilution from copious amounts of rainwater). Bio decay will also add nitric acid which will further lower pH (providing you do not over clean the substrate). Because of this, do not try and lower GH (only carbonate hardness), calcium in particular as this is still an important element for osmoregulation in Discus and other Amazonian fish as well (calcium aids in a reducing Redox as well!).
As noted earlier, Do NOT use pH lowering products such as pH Down (or pH Up) as these will just cause a roller coaster effect on your pH. In fact, I have seen both from my use or a client adding pH Down (or similar products); the pH drops as desired only to rebound very quickly due to alkaline buffers often present in most tap water sources (depending upon your location of course). This rebound can quickly shock and stress delicate fish since as noted earlier a change in pH up or down of just one point is a tenfold change.
Better to concentrate on a stable KH and use blended or re-mineralized RO water along with pH buffering products such as Indian Almond Leaves, Pillow Moss, Peat, and Driftwood, or at the very least use true acid buffering products such as SeaChem’s Acid Buffer (which should be always used in combination with an alkaline buffer, even baking soda.
Product Resources:
• Atison’s Betta Spa
• AAP Premium Wonder Shells
• SeaChem Acid Buffer; for Quick pH/KH Reduction
• Pillow/Frog Moss; Natural Aquatic Low pH Buffer
Other products that are popular for softening water, lowering pH, and for creating an Amazon or Breeding environment include black water tonics and Atison’s SPA. I personally do not care for the old black water remedies as I find them to mostly work like the proverbial “chicken soup” placebo effect when put to actual tests and in fact I have seen harmful anaerobic bacteria added from these remedies.
Natural tannin products such as Pillow Moss along with a constant supply of calcium ions provided by a second product would be my first choice (based on the benefits of constant mineral ions/cations as pointed out earlier in the article).
Another choice is Atison’s Spa (which also contains calcium). However, this product sometimes fools users who understand the need for calcium, but miss the even more important fact that while Discus, Bettas, Rams and other Amazon/SE Asia Fish need small amounts of calcium, what is more important is the regular supply of positive calcium ion, albeit in small amounts. For this reason, I still strongly recommend a small but regular addition of mineral supplements whether liquid or in dissolving block form.
As to Atison’s SPA, this is growing in popularity among Betta enthusiasts due to mega internet site promotions. However, it uses almond leaves that are very refined which causes the loss of potency as compared to the superior pillow moss or the use of simply raw Almond Leaves. The Betta breeders in LA I know that previously used Bio Lif (now unfortunately discontinued) and now simply use raw Almond leaves or Pillow Moss along with constant mineral supplements and get good results, however these guys are not active in Betta or Discus groups/forums, so these methods are not nearly as well publicized as the use of Atison’s SPA.
The bottom line as per Atison's Betta Spa, is this is best used in small tanks/bowls where use of more advanced methods is often difficult.
Other choices include the before mentioned Pillow Moss, Peat, Driftwood pieces, etc. supplemented by a separate addition of minerals such as with Wonder Shells or a drip method of liquid calcium with products such as SeaChem Replenish in a volume of water which will add a constant supply of important mineral cations that are essential for fish or even water changes using high calcium water.
Finally, it is important as you also want to note that the Amazon River starts high in the Andes Mountains where it picks up a lot of minerals only to be buffer “down” by organics such as peat and bio-decay (as well as dilution from copious amounts of rainwater). Bio decay will also add nitric acid which will further lower pH (providing you do not over clean the substrate). Because of this, do not try and lower GH (only carbonate hardness), calcium in particular as this is still an important element for osmoregulation in Discus and other Amazonian fish as well (calcium aids in a reducing Redox as well!).
As noted earlier, Do NOT use pH lowering products such as pH Down (or pH Up) as these will just cause a roller coaster effect on your pH. In fact, I have seen both from my use or a client adding pH Down (or similar products); the pH drops as desired only to rebound very quickly due to alkaline buffers often present in most tap water sources (depending upon your location of course). This rebound can quickly shock and stress delicate fish since as noted earlier a change in pH up or down of just one point is a tenfold change.
Better to concentrate on a stable KH and use blended or re-mineralized RO water along with pH buffering products such as Indian Almond Leaves, Pillow Moss, Peat, and Driftwood, or at the very least use true acid buffering products such as SeaChem’s Acid Buffer (which should be always used in combination with an alkaline buffer, even baking soda.
Product Resources:
• Atison’s Betta Spa
• AAP Premium Wonder Shells
• SeaChem Acid Buffer; for Quick pH/KH Reduction
• Pillow/Frog Moss; Natural Aquatic Low pH Buffer
Facts About Minerals
All fish require calcium and other mineral cations, including discus, bettas, tetras and other soft water fish. In fact, the lack of electrolytes and calcium is a MAJOR problem for poor health in bettas and discus.
Good water management should ALWAYS insure good electrolyte levels along with water changes and other aquatic husbandry practices. Use of water conditioners as well mineral blocks can help insure this.
Calcium and other minerals added to fish food made from poor quality ingredients are usually not adequate for proper health (some studies show these added minerals block absorption). Calcium and other minerals in the water (especially for marine organisms) as well as foods high in natural minerals such as Spirulina] are also important.
Reference:
• The benefits of Spirulina Algae
Although the addition of calcium and electrolytes may initially stress fish that have had low electrolyte levels, this does NOT prove these elements detrimental any more than changing water in an aquarium that is long overdue for a water change (this too may initially stress fish). A GH of 20 dGH (over 350 ppm) is easily tolerated by ALL fish especially when calcium makes up the majority of this GH. Separate calcium and magnesium tests can verify this.
Although RO or DI water may be very clean and pure, by itself it can be VERY detrimental to fish without the addition of electrolytes and essential minerals such as calcium. If you use it, make sure you reconstitute it. I generally use Reverse Osmosis (RO) water blended with tap water (for soft/low pH fish such as discus), then adding a Wonder Shells, Replenish, SeaChem Trace or similar for added trace elements as well as alkaline and acid buffers). It is even more important to reconstitute 100% RO or DI.
Please see the Chart in the KH Buffers Section for further information about the use of Alkaline and Acid Buffers together and at the correct ratios.
"Drinking Water" sold in stores is usually just RO water with SOME minerals added for “taste” and is FAR from appropriate for aquarium use. Usually, true spring water is OK. However, RO or DI water is fine for marine use (in fact I recommend it) as salt mixes add all the trace elements needed.
Product Resource:
• TMC Professional Reverse Osmosis Systems for Aquariums
It is possible to have adequate calcium and electrolytes without raising pH to a harmful level for discus, bettas and other fish that generally prefer low pH water (many discus breeders in LA keep their discus pH higher than in the wild anyway).
Good water management should ALWAYS insure good electrolyte levels along with water changes and other aquatic husbandry practices. Use of water conditioners as well mineral blocks can help insure this.
Calcium and other minerals added to fish food made from poor quality ingredients are usually not adequate for proper health (some studies show these added minerals block absorption). Calcium and other minerals in the water (especially for marine organisms) as well as foods high in natural minerals such as Spirulina] are also important.
Reference:
• The benefits of Spirulina Algae
Although the addition of calcium and electrolytes may initially stress fish that have had low electrolyte levels, this does NOT prove these elements detrimental any more than changing water in an aquarium that is long overdue for a water change (this too may initially stress fish). A GH of 20 dGH (over 350 ppm) is easily tolerated by ALL fish especially when calcium makes up the majority of this GH. Separate calcium and magnesium tests can verify this.
Although RO or DI water may be very clean and pure, by itself it can be VERY detrimental to fish without the addition of electrolytes and essential minerals such as calcium. If you use it, make sure you reconstitute it. I generally use Reverse Osmosis (RO) water blended with tap water (for soft/low pH fish such as discus), then adding a Wonder Shells, Replenish, SeaChem Trace or similar for added trace elements as well as alkaline and acid buffers). It is even more important to reconstitute 100% RO or DI.
Please see the Chart in the KH Buffers Section for further information about the use of Alkaline and Acid Buffers together and at the correct ratios.
"Drinking Water" sold in stores is usually just RO water with SOME minerals added for “taste” and is FAR from appropriate for aquarium use. Usually, true spring water is OK. However, RO or DI water is fine for marine use (in fact I recommend it) as salt mixes add all the trace elements needed.
Product Resource:
• TMC Professional Reverse Osmosis Systems for Aquariums
It is possible to have adequate calcium and electrolytes without raising pH to a harmful level for discus, bettas and other fish that generally prefer low pH water (many discus breeders in LA keep their discus pH higher than in the wild anyway).
Quick Tips for Adding Buffers, etc.
Daily Dosing: For those who prefer perfect KH and interaction of Acid & Alkaline Buffers, this might be for you. Adding Acid and Alkaline every morning can provide the chemical reaction to add additional CO2 for plant enthusiasts. However, you still need to experiment to find the sweet spot in your aquarium chemistry that does not result in too high or low KH by the next morning and adjust ratios accordingly.
Weekly or Bi-Weekly Dosing: This is the most common and is the method I chose with the vast majority of my clients. Simply figure the amount of Alkaline Buffer needed to maintain the desired KH for this time period. For example, if 150 ppm is desired adding buffers to 170 ppm and then finding your KH at 130 two weeks later is generally acceptable as long as adding buffers at this time does not cause a pH rise of more than .5. Except for Amazon River or similar aquariums, generally Acid Buffers are not called for in this method.
Fast pH/KH Drops between dosing: Look for too much decomposing mulm, peat, driftwood, pillow moss, over feeding, overly saturated canister filters, etc. Or simply too much Acid Buffer (if used).
Fast pH/KH Rises between dosing: As noted more in depth in this article, this can simply gas out of new water, so allow at least an hour before a pH test after adding. Use of decorations that might have carbonates contained therein, such as Coral Skeletons in freshwater. Use of 10%, 25%, or even higher amounts of RO water may be a solution rather than chasing the exact blend of Acid & Alkaline Buffers.
Also overuse of alkaline buffers can have this result too.
Desired KH raises pH to undesirable Level: Let's say for example you have goldfish and would like to keep your KH at 150 ppm. However, your pH remains at 8.2, what do you do? For one you could simply accept this pH as although 8.0 or less is considered more desirable for goldfish, as I have pointed out in this article a more staple pH and constant KH is more important. Two, you could lower your KH and accept the pH this brings. Or three you could use 25% (or some other amount) of RO or DI water to cut the minerals and carbonates in the tap/well water being used and then adjust buffers to 150 ppm KH and likely your pH will be a little lower.
Test, Test, & Test: While I wish it were possible to give an exact measurement for everyone, I cannot. The FACT is aquarium keeping is a science and EVERY tank is unique for a multitude of reasons and hopefully with a reading (or maybe 2 or 3 readings) of this article you will understand how best to keep your aquarium.
Weekly or Bi-Weekly Dosing: This is the most common and is the method I chose with the vast majority of my clients. Simply figure the amount of Alkaline Buffer needed to maintain the desired KH for this time period. For example, if 150 ppm is desired adding buffers to 170 ppm and then finding your KH at 130 two weeks later is generally acceptable as long as adding buffers at this time does not cause a pH rise of more than .5. Except for Amazon River or similar aquariums, generally Acid Buffers are not called for in this method.
Fast pH/KH Drops between dosing: Look for too much decomposing mulm, peat, driftwood, pillow moss, over feeding, overly saturated canister filters, etc. Or simply too much Acid Buffer (if used).
Fast pH/KH Rises between dosing: As noted more in depth in this article, this can simply gas out of new water, so allow at least an hour before a pH test after adding. Use of decorations that might have carbonates contained therein, such as Coral Skeletons in freshwater. Use of 10%, 25%, or even higher amounts of RO water may be a solution rather than chasing the exact blend of Acid & Alkaline Buffers.
Also overuse of alkaline buffers can have this result too.
Desired KH raises pH to undesirable Level: Let's say for example you have goldfish and would like to keep your KH at 150 ppm. However, your pH remains at 8.2, what do you do? For one you could simply accept this pH as although 8.0 or less is considered more desirable for goldfish, as I have pointed out in this article a more staple pH and constant KH is more important. Two, you could lower your KH and accept the pH this brings. Or three you could use 25% (or some other amount) of RO or DI water to cut the minerals and carbonates in the tap/well water being used and then adjust buffers to 150 ppm KH and likely your pH will be a little lower.
Test, Test, & Test: While I wish it were possible to give an exact measurement for everyone, I cannot. The FACT is aquarium keeping is a science and EVERY tank is unique for a multitude of reasons and hopefully with a reading (or maybe 2 or 3 readings) of this article you will understand how best to keep your aquarium.
Freshwater GH, KH, pH; Basic- Chemistry Suggestions
I should note immediately that these suggestions are NOT meant to be the only way, these are simply suggestions based on experience and good science/research. What is most important is not the how, but the FACT that many mineral cations are essential for fish health from Freshwater Discus to Marine Reefs.
Often experienced aquarists do not take extra steps to provide these (a mistake often made when utilizing the "Walstad Method" of aquarium keeping), yet they inadvertently provide essential positive mineral ions and carbonates via good aquatic husbandry, substrate choices and more.
Reference:
• Planted Aquarium Care; Filters
However, it is not correct to state that just because one does not take extra measures, others should not as well. It is noteworthy that even experienced aquarists may not be maintaining the best environment possible, as statements such as: “I do not maintain or check my positive mineral ions and my fish are fine” prove absolutely nothing! Good science states otherwise to such statements, the reason being that many fish adapt to poor environments but do not thrive as well as they could.
Also as noted earlier in this article (the KH section); there are NO EXACT ratios as each aquarium is very unique; so, testing is 100% required and even then, testing should be performed hours or even a day later after addition of mineral and/or buffering products to allow the chemistry to "settle". With the use of peat, pillow moss, Indian almond leaves (such as Atison's Betta Spa), driftwood and similar; this "settling in" may take weeks.
Once you establish a "sweet spot" for your unique aquarium environment, use the blends ratio numbers that work for you! This may mean a drip system, mineral blocks, daily small doses of buffers (so as to have constant reaction and very little change in KH), or weekly or even bi-weekly additions of buffers as I usually did with my aquarium maintenance clients (as I could not be at my clients every day, so the buffers and mineral replenishments had to be adequate for the gap between visits).
Basic Widely Mixed Community, including Tetras, Gouramis, Catfish, Loaches, Danios, Livebearers, many Cichlids, etc.:
• Aim for a pH that is stable (not an exact number) in the range of 6.6 to 7.8.
• A KH of anywhere from 80 to 150 is generally good and this can be maintained with products such as Sea Chem Alkaline Buffer. If tap water is high pH/KH I would recommend using 10-20% Reverse Osmosis Water and/or products such as Frog Moss or Mango/Driftwood to counter the high pH/KH. If your water source KH is low (tap water, etc.), adding buffers to your tap or other water source to bring it up to the desired KH should be performed with every water change. Small amounts of KH buffers will likely need to be added in intervals in between water changes based on KH tests.
• GH is best between 150- 300, although this can be misleading in that CaCO3+ (& other positive mineral ions) can be depleted and your GH still reads high. The use of Wonder Shells or the slow drip addition of powdered or liquid mineral supplements such as AAP/SeaChem Trace or Replenish can help with this (simply adding these liquid or dry mineral supplements on a regular basis can work well too). 1-5% water changes daily can help as well provided your water source has these important mineral cations. If salt is necessary with lower pH mixed aquariums, I generally just use water softener salt or any plain salt.
Soft Water Low pH, KH Amazon River, SE Asia or West African Aquarium:
• As with the previous section (Basic mixed aquarium), aim for a pH that is stable, do Not chase pH. Chasing pH is all too often performed by inexperienced Discus, Angelfish, etc. keepers. In fact, I know of two very successful commercial Discus breeders that maintained their Discus pH at about 7.0-7.4. This said, I personally have found a pH between 6.0 to 7.2 works well for most SA Cichlids from Discus to Ram Cichlids, so I suggest stabilizing at a number that is easiest in this range to achieve using methods described earlier in this article and do not chase the pH otherwise.
Reference:
• Nitrogen Cycle; Low pH and Nitrification
From the above cited article: "It is also noteworthy that the primary nitrifying bacteria are affected by pH. Nitrification involving AOB & NOB bacteria is different at pH levels of above 7.0 versus below 6.0.
Toxic Ammonia (NH3) changes to ammonium under 6.0 and ammonium (nontoxic NH4) switches back to toxic NH3 over 7.0 What is important, is nitrification rates are rapidly depressed as the pH is raised above 7.0 from pH levels under 6.0 until the nitrification process re-establishes itself at the higher pH."
• KH is still important with these soft water fish (maybe not as important as with a Rift Lake Cichlid). The reason is to prevent pH spikes (usually down from other methods used for a soft water environment such as peat or even simply from biological waste breakdown). Generally, utilize KH buffers (such as SeaChem Alkaline Buffer or even Baking Soda) to maintain a KH of about 50-80 ppm if necessary.
The use of natural acid buffers such as Frog Moss, Peat, Indian Almond Leaves, and/or Driftwood should also be employed to balance the pH and react with the carbonates provided by the KH, which in turn provides CO2 for live plants that are often kept in this type of aquarium. Generally Almond Leaves, Driftwood, and many forms of Peat are "Slow" acid buffers, while Frog Moss tends to be a faster natural acid buffer. The use of a chemical "fast" acid buffer such SeaChem Acid Buffer should be considered at set up and for water changes (or in between if there are issues with pH climbing or if more CO2 is needed (a KH buffer must be used to cause the reaction that produces CO2).
• GH: The main importance with soft water fish is not GH per say, rather the very important positive mineral ions (such as Calcium+++ or Magnesium+++) that have been proven to improve fish health and prevent diseases, such as Columnaris.
Reference:
• Prevention and treatment of Columnaris
Since GH test kits give only half the “picture” (a Redox Meter or even a simple Methylene Blue Redox test can help), a target GH does not necessarily mean there are adequate mineral cations. However, a GH of 100-150 that is regularly replenished should aid in providing these mineral ions.
For these soft water aquariums the use Reverse Osmosis water (or partial RO water), then the use of a more measured dosing mineral/electrolyte product such as SeaChem Replenish to add these important minerals without making the aquarium GH climb more than desired (although as noted earlier, a GH over 150 is not the problem many soft water fish keepers make it out to be), then the possible addition products such as Wonder Shells or similar products (such as utilizing a drip of or AragaMix) at 1/4 dose or 1/4 block can aid in providing constant supply of important mineral cations.
Please see the section earlier in this article for much more about
• Amazon River/SE Asia/West African Water Chemistry/pH, GH, KH
Goldfish/Koi: Aim for a pH that is stable, as before, not an exact number but in a range of 7.2 to 8.0 should be fine.
• A KH of anywhere from 100 to 200 is generally good and this can be maintained with products such as Sea Chem Alkaline or Marine Buffer. With Goldfish, the use of a “complete” buffer such as the SeaChem Malawi may be useful for a more balanced GH/KH, however in hard water areas the regular alkaline buffer all that is needed for goldfish. If your water source KH is low (tap water, etc.), adding buffers to your tap or other water source to bring it up to the desired KH should be performed with every water change. Small amounts of KH buffers will likely need to be added in intervals in between water changes based on KH tests.
• GH is best between 200- 300, although again this can be misleading in that CaCO3+ (& other mineral cations) can be depleted and your GH still reads high. The use of Wonder Shells can create a constant supply of necessary positive mineral ions or the use of either a drip/dosing or simply scheduled use of liquid or dry mineral supplements should be employed to supply these essential mineral ions.
Livebearers: Aim for a pH that is stable, as before, not an exact number but in a range of 7.2 to 8.4 should be fine.
• A KH of anywhere from 150 to 250 is generally good and this can be maintained with products such as Sea Chem Malawi Buffer. The difference from the mixed community tank is that I recommend using more advanced buffer that includes minerals such as Marine or Malawi Buffer, although you obviously want to test your KH to find the “sweet spot” in the amount needed to fit your bio load and tap water adjustments. Generally Acid Buffers are not necessary for livebearers as the normal bio processes produce more than enough acids that react with alkaline buffers and supplementing with acid buffering products would require even more Alkaline Buffers for higher pH/GH preferring fish.
If your water source KH is low (tap water, etc.), adding buffers to your tap or other water source to bring it up to the desired KH should be performed with every water change. Small amounts of KH buffers will likely need to be added in intervals in between water changes based on KH tests.
• GH generally between 250- 350 (even as high as 500), although again this can be misleading in that CaCO3+ (& other mineral cations) can be depleted and your GH still reads high. The use of Wonder Shells can create a constant supply of necessary positive mineral ions and the use of crushed coral gravel can supplement this further if desired. AAP Cichlid Salt is also suggested to provide a balance of sodium chloride salt and other essential minerals and can be used with or without our mineral supplements (such as the before mentioned Wonder Shells); testing your KH & GH before and after water changes as well as intermittently will help determine the amount to use.
Rift Lake Cichlids: Similar to livebearers, except many aspects should be often higher yet in alkalinity/pH, especially for Lake Tanganyika Cichlids.
• Again, aim for a pH that is stable, as before, not an exact number but in a range of 7.8 to 8.4 should be fine for most Malawi or Victoria Cichlids and 8.0 and higher for Lake Tanganyika Cichlids.
• A similar approach to livebearers except the goal is for a slightly higher KH (more so with Tanganyika Cichlids) with a KH of anywhere from 150 to 300 being generally good and this can be maintained with products such as Sea Chem Malawi Buffer. If your water source KH is low (tap water, etc.), adding buffers to your tap or other water source to bring it up to the desired KH should be performed with every water change. Small amounts of KH buffers will likely need to be added in intervals in between water changes based on KH tests.
• GH is best between 250- 350 (or even higher) and as stated before, do NOT depend on your GH test kit to give you the full picture of the yet even more important for Rift Lake Cichlids mineral cations. These large lakes have a large in flow and reserve of these ESSENTIAL positive mineral ions that CANNOT be easily duplicated in an aquarium, even with the use of crushed coral gravel (although this is certainly a good start). The use of an even more fine oolite coral sand is even better for supplying these mineral cations important for osmoregulation, Redox Balance and in the end good fish health. The use of Wonder Shells can help with a steadier supply of these mineral positive electrolytes.
Regular water changes, especially with high mineral water (often tap water is great for this) should NEVER be overlooked as well. The use of Sea Chem Salt can provide both sodium chloride salt and additional minerals and can be used with Wonder Shell, AAP/SeaChem Replenish or Trace or similar mineral supplements or by itself.
List of Products & their suggested purchase source for Aquarium Chemistry by Category (Please note that this is not intended as a complete list of products, however similar products can be found by drawing conclusions from this list:
Alkaline Buffers:
• SeaChem Malawi Buffer
• SeaChem Marine Buffer
• SeaChem Alkaline Buffer
• SeaChem Cichlid Salt
• Baking Soda
Acid Buffers:
• SeaChem Acid Buffer
• Frog Moss (Pillow Moss)
• Bio Lif (Indian Almond Leaves)
• Atison’s Spa (Indian Almond Leaves based conditioner)
• Peat
• Driftwood (or Driftwood pieces in filter)
Mineral Replenishment:
• Wonder Shells (unique AAP)
• AAP/SeaChem Replenish
• SeaChem Fresh Trace
• Aragamight
• AAP/SeaChem Cichlid Salt (this also adds Sodium Chloride Salt)
• Oolite coral sand (including for FSB Filters)
• Crushed Coral (a very poor source)
Often experienced aquarists do not take extra steps to provide these (a mistake often made when utilizing the "Walstad Method" of aquarium keeping), yet they inadvertently provide essential positive mineral ions and carbonates via good aquatic husbandry, substrate choices and more.
Reference:
• Planted Aquarium Care; Filters
However, it is not correct to state that just because one does not take extra measures, others should not as well. It is noteworthy that even experienced aquarists may not be maintaining the best environment possible, as statements such as: “I do not maintain or check my positive mineral ions and my fish are fine” prove absolutely nothing! Good science states otherwise to such statements, the reason being that many fish adapt to poor environments but do not thrive as well as they could.
Also as noted earlier in this article (the KH section); there are NO EXACT ratios as each aquarium is very unique; so, testing is 100% required and even then, testing should be performed hours or even a day later after addition of mineral and/or buffering products to allow the chemistry to "settle". With the use of peat, pillow moss, Indian almond leaves (such as Atison's Betta Spa), driftwood and similar; this "settling in" may take weeks.
Once you establish a "sweet spot" for your unique aquarium environment, use the blends ratio numbers that work for you! This may mean a drip system, mineral blocks, daily small doses of buffers (so as to have constant reaction and very little change in KH), or weekly or even bi-weekly additions of buffers as I usually did with my aquarium maintenance clients (as I could not be at my clients every day, so the buffers and mineral replenishments had to be adequate for the gap between visits).
Basic Widely Mixed Community, including Tetras, Gouramis, Catfish, Loaches, Danios, Livebearers, many Cichlids, etc.:
• Aim for a pH that is stable (not an exact number) in the range of 6.6 to 7.8.
• A KH of anywhere from 80 to 150 is generally good and this can be maintained with products such as Sea Chem Alkaline Buffer. If tap water is high pH/KH I would recommend using 10-20% Reverse Osmosis Water and/or products such as Frog Moss or Mango/Driftwood to counter the high pH/KH. If your water source KH is low (tap water, etc.), adding buffers to your tap or other water source to bring it up to the desired KH should be performed with every water change. Small amounts of KH buffers will likely need to be added in intervals in between water changes based on KH tests.
• GH is best between 150- 300, although this can be misleading in that CaCO3+ (& other positive mineral ions) can be depleted and your GH still reads high. The use of Wonder Shells or the slow drip addition of powdered or liquid mineral supplements such as AAP/SeaChem Trace or Replenish can help with this (simply adding these liquid or dry mineral supplements on a regular basis can work well too). 1-5% water changes daily can help as well provided your water source has these important mineral cations. If salt is necessary with lower pH mixed aquariums, I generally just use water softener salt or any plain salt.
Soft Water Low pH, KH Amazon River, SE Asia or West African Aquarium:
• As with the previous section (Basic mixed aquarium), aim for a pH that is stable, do Not chase pH. Chasing pH is all too often performed by inexperienced Discus, Angelfish, etc. keepers. In fact, I know of two very successful commercial Discus breeders that maintained their Discus pH at about 7.0-7.4. This said, I personally have found a pH between 6.0 to 7.2 works well for most SA Cichlids from Discus to Ram Cichlids, so I suggest stabilizing at a number that is easiest in this range to achieve using methods described earlier in this article and do not chase the pH otherwise.
Reference:
• Nitrogen Cycle; Low pH and Nitrification
From the above cited article: "It is also noteworthy that the primary nitrifying bacteria are affected by pH. Nitrification involving AOB & NOB bacteria is different at pH levels of above 7.0 versus below 6.0.
Toxic Ammonia (NH3) changes to ammonium under 6.0 and ammonium (nontoxic NH4) switches back to toxic NH3 over 7.0 What is important, is nitrification rates are rapidly depressed as the pH is raised above 7.0 from pH levels under 6.0 until the nitrification process re-establishes itself at the higher pH."
• KH is still important with these soft water fish (maybe not as important as with a Rift Lake Cichlid). The reason is to prevent pH spikes (usually down from other methods used for a soft water environment such as peat or even simply from biological waste breakdown). Generally, utilize KH buffers (such as SeaChem Alkaline Buffer or even Baking Soda) to maintain a KH of about 50-80 ppm if necessary.
The use of natural acid buffers such as Frog Moss, Peat, Indian Almond Leaves, and/or Driftwood should also be employed to balance the pH and react with the carbonates provided by the KH, which in turn provides CO2 for live plants that are often kept in this type of aquarium. Generally Almond Leaves, Driftwood, and many forms of Peat are "Slow" acid buffers, while Frog Moss tends to be a faster natural acid buffer. The use of a chemical "fast" acid buffer such SeaChem Acid Buffer should be considered at set up and for water changes (or in between if there are issues with pH climbing or if more CO2 is needed (a KH buffer must be used to cause the reaction that produces CO2).
• GH: The main importance with soft water fish is not GH per say, rather the very important positive mineral ions (such as Calcium+++ or Magnesium+++) that have been proven to improve fish health and prevent diseases, such as Columnaris.
Reference:
• Prevention and treatment of Columnaris
Since GH test kits give only half the “picture” (a Redox Meter or even a simple Methylene Blue Redox test can help), a target GH does not necessarily mean there are adequate mineral cations. However, a GH of 100-150 that is regularly replenished should aid in providing these mineral ions.
For these soft water aquariums the use Reverse Osmosis water (or partial RO water), then the use of a more measured dosing mineral/electrolyte product such as SeaChem Replenish to add these important minerals without making the aquarium GH climb more than desired (although as noted earlier, a GH over 150 is not the problem many soft water fish keepers make it out to be), then the possible addition products such as Wonder Shells or similar products (such as utilizing a drip of or AragaMix) at 1/4 dose or 1/4 block can aid in providing constant supply of important mineral cations.
Please see the section earlier in this article for much more about
• Amazon River/SE Asia/West African Water Chemistry/pH, GH, KH
Goldfish/Koi: Aim for a pH that is stable, as before, not an exact number but in a range of 7.2 to 8.0 should be fine.
• A KH of anywhere from 100 to 200 is generally good and this can be maintained with products such as Sea Chem Alkaline or Marine Buffer. With Goldfish, the use of a “complete” buffer such as the SeaChem Malawi may be useful for a more balanced GH/KH, however in hard water areas the regular alkaline buffer all that is needed for goldfish. If your water source KH is low (tap water, etc.), adding buffers to your tap or other water source to bring it up to the desired KH should be performed with every water change. Small amounts of KH buffers will likely need to be added in intervals in between water changes based on KH tests.
• GH is best between 200- 300, although again this can be misleading in that CaCO3+ (& other mineral cations) can be depleted and your GH still reads high. The use of Wonder Shells can create a constant supply of necessary positive mineral ions or the use of either a drip/dosing or simply scheduled use of liquid or dry mineral supplements should be employed to supply these essential mineral ions.
Livebearers: Aim for a pH that is stable, as before, not an exact number but in a range of 7.2 to 8.4 should be fine.
• A KH of anywhere from 150 to 250 is generally good and this can be maintained with products such as Sea Chem Malawi Buffer. The difference from the mixed community tank is that I recommend using more advanced buffer that includes minerals such as Marine or Malawi Buffer, although you obviously want to test your KH to find the “sweet spot” in the amount needed to fit your bio load and tap water adjustments. Generally Acid Buffers are not necessary for livebearers as the normal bio processes produce more than enough acids that react with alkaline buffers and supplementing with acid buffering products would require even more Alkaline Buffers for higher pH/GH preferring fish.
If your water source KH is low (tap water, etc.), adding buffers to your tap or other water source to bring it up to the desired KH should be performed with every water change. Small amounts of KH buffers will likely need to be added in intervals in between water changes based on KH tests.
• GH generally between 250- 350 (even as high as 500), although again this can be misleading in that CaCO3+ (& other mineral cations) can be depleted and your GH still reads high. The use of Wonder Shells can create a constant supply of necessary positive mineral ions and the use of crushed coral gravel can supplement this further if desired. AAP Cichlid Salt is also suggested to provide a balance of sodium chloride salt and other essential minerals and can be used with or without our mineral supplements (such as the before mentioned Wonder Shells); testing your KH & GH before and after water changes as well as intermittently will help determine the amount to use.
Rift Lake Cichlids: Similar to livebearers, except many aspects should be often higher yet in alkalinity/pH, especially for Lake Tanganyika Cichlids.
• Again, aim for a pH that is stable, as before, not an exact number but in a range of 7.8 to 8.4 should be fine for most Malawi or Victoria Cichlids and 8.0 and higher for Lake Tanganyika Cichlids.
• A similar approach to livebearers except the goal is for a slightly higher KH (more so with Tanganyika Cichlids) with a KH of anywhere from 150 to 300 being generally good and this can be maintained with products such as Sea Chem Malawi Buffer. If your water source KH is low (tap water, etc.), adding buffers to your tap or other water source to bring it up to the desired KH should be performed with every water change. Small amounts of KH buffers will likely need to be added in intervals in between water changes based on KH tests.
• GH is best between 250- 350 (or even higher) and as stated before, do NOT depend on your GH test kit to give you the full picture of the yet even more important for Rift Lake Cichlids mineral cations. These large lakes have a large in flow and reserve of these ESSENTIAL positive mineral ions that CANNOT be easily duplicated in an aquarium, even with the use of crushed coral gravel (although this is certainly a good start). The use of an even more fine oolite coral sand is even better for supplying these mineral cations important for osmoregulation, Redox Balance and in the end good fish health. The use of Wonder Shells can help with a steadier supply of these mineral positive electrolytes.
Regular water changes, especially with high mineral water (often tap water is great for this) should NEVER be overlooked as well. The use of Sea Chem Salt can provide both sodium chloride salt and additional minerals and can be used with Wonder Shell, AAP/SeaChem Replenish or Trace or similar mineral supplements or by itself.
List of Products & their suggested purchase source for Aquarium Chemistry by Category (Please note that this is not intended as a complete list of products, however similar products can be found by drawing conclusions from this list:
Alkaline Buffers:
• SeaChem Malawi Buffer
• SeaChem Marine Buffer
• SeaChem Alkaline Buffer
• SeaChem Cichlid Salt
• Baking Soda
Acid Buffers:
• SeaChem Acid Buffer
• Frog Moss (Pillow Moss)
• Bio Lif (Indian Almond Leaves)
• Atison’s Spa (Indian Almond Leaves based conditioner)
• Peat
• Driftwood (or Driftwood pieces in filter)
Mineral Replenishment:
• Wonder Shells (unique AAP)
• AAP/SeaChem Replenish
• SeaChem Fresh Trace
• Aragamight
• AAP/SeaChem Cichlid Salt (this also adds Sodium Chloride Salt)
• Oolite coral sand (including for FSB Filters)
• Crushed Coral (a very poor source)
Summary
There is much scientific practical evidence to support the need of Calcium, a healthy KH, GH, and Electrolytes in a Freshwater Aquarium. This is more widely known for marine aquariums, however these important parameters and the interaction they have on fish health in freshwater are often missed. The minerals often found in GH (Calcium in particular) have also shown to be effective for BOTH prevention and treatment of Hole in the Head (HITH) in many cichlids.
Many freshwater aquarists will often concern themselves with a certain pH all the while missing the importance KH plays in the stability of pH even in Amazon River tanks (where low pH buffers should also be employed).
The need for calcium by all creatures is well documented, especially in studies outside the aquatics industry/hobby. Calcium and other elements found in a healthy GH are important for osmoregulation in ALL freshwater fish and even plants (saltwater organisms too). These elements also aid in a healthy Redox Balance as well. The addition of pH up or pH down products is often counterproductive vs. using products that stabilize your aquariums KH and GH, these products often result in a bouncing pH.
Products such as Wonder Shells will help with your calcium, magnesium, GH of your aquarium (especially important CaCO3+ which are quickly depleted, even when GH tests kits show abundant Calcium/minerals the beneficial positive ions may be depleted). I prefer this over crushed coral for adding calcium, magnesium and electrolytes as from my experience (or SeaChem Replenish/Trace, Oolitic Sand in an FSB Filter, or Aragamix). Wonder Shells also are available in a unique medicated version for control of aquarium ich and fungus.
Many freshwater aquarists will often concern themselves with a certain pH all the while missing the importance KH plays in the stability of pH even in Amazon River tanks (where low pH buffers should also be employed).
The need for calcium by all creatures is well documented, especially in studies outside the aquatics industry/hobby. Calcium and other elements found in a healthy GH are important for osmoregulation in ALL freshwater fish and even plants (saltwater organisms too). These elements also aid in a healthy Redox Balance as well. The addition of pH up or pH down products is often counterproductive vs. using products that stabilize your aquariums KH and GH, these products often result in a bouncing pH.
Products such as Wonder Shells will help with your calcium, magnesium, GH of your aquarium (especially important CaCO3+ which are quickly depleted, even when GH tests kits show abundant Calcium/minerals the beneficial positive ions may be depleted). I prefer this over crushed coral for adding calcium, magnesium and electrolytes as from my experience (or SeaChem Replenish/Trace, Oolitic Sand in an FSB Filter, or Aragamix). Wonder Shells also are available in a unique medicated version for control of aquarium ich and fungus.
Videos
|
|
|
|